2Department of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
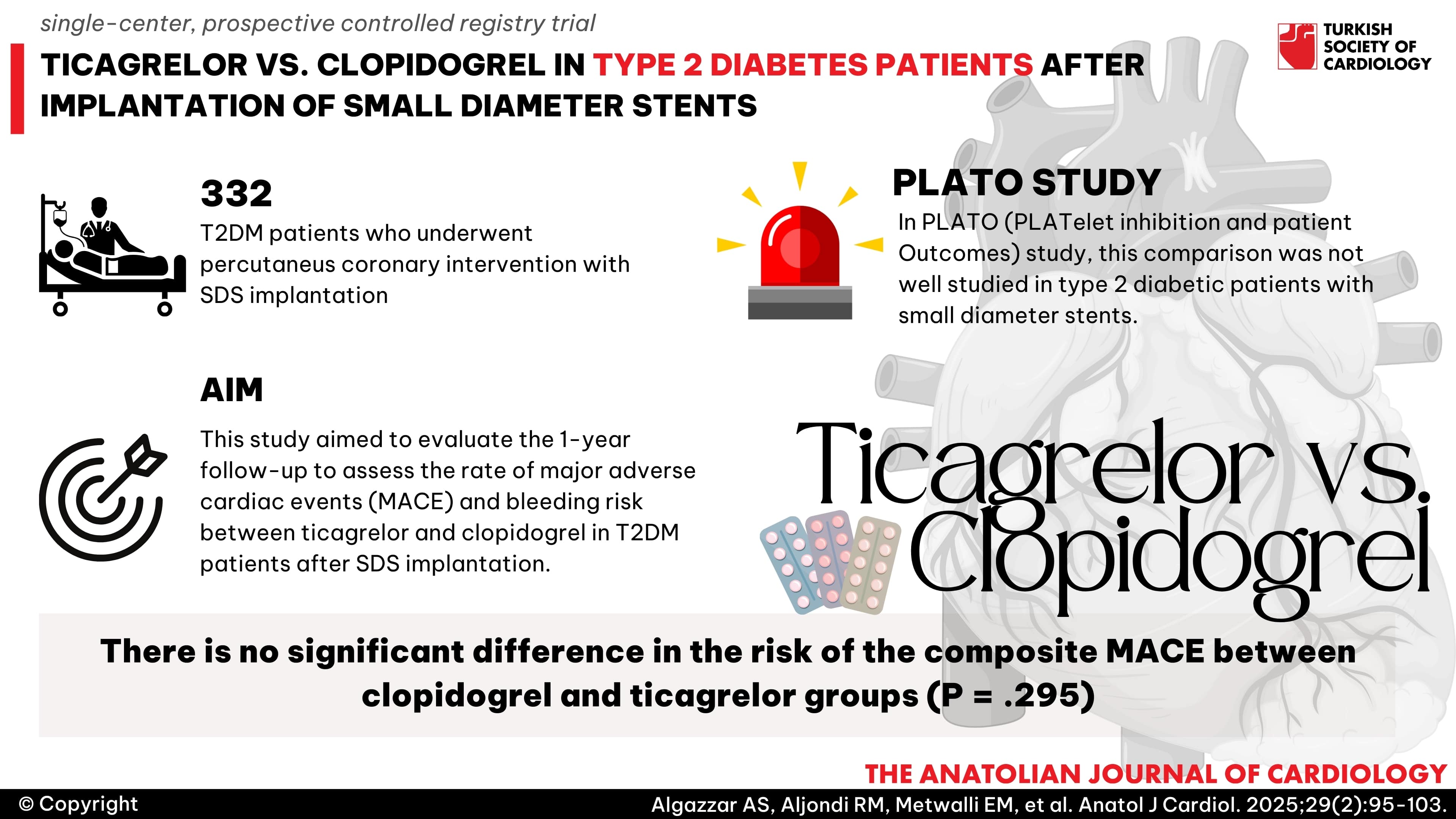
Abstract
Background: Type 2 diabetes mellitus (T2DM) patients with small-diameter stents (SDS), that are equal to or less than 2.5 mm in diameter, face increased risks of restenosis and complications. This study aimed to evaluate the 1-year follow-up to assess the rate of major adverse cardiac events (MACE) and bleeding risk between ticagrelor and clopidogrel in T2DM patients after SDS implantation.
Methods: The study was a single-center, prospective controlled registry trial, which included 332 T2DM patients who underwent percutaneous coronary intervention with SDS implantation. Follow-up was conducted for 1 year.
Results: Following propensity score matching, the 1-year analysis revealed no significant difference in the risk of the composite MACE between clopidogrel and ticagrelor groups (P = .295). Male gender, history of ischemic heart disease, ejection fraction (EF), coronary lesion type, and chronic kidney disease (CKD) were identified as potential predictors for the composite endpoint. In a sub-analysis of CKD patients, the 12-month rates of composites of cardiac death (CD), myocardial infarction (MI), stroke, and target vessel revascularization (TVR) were lower in the ticagrelor group than in the clopidogrel group (P = .024). However, the ticagrelor group was associated with a higher rate of bleeding compared to the clopidogrel group (20% vs. 9%) (P = .041).
Conclusion: Our study demonstrated that ticagrelor did not show improvement in the composite of CD, MI, stroke, TVR, or the risk of bleeding events defined by the BARC criteria in patients with T2DM and SDS compared with clopidogrel emphasizing the importance of individualized treatment decisions based on patient characteristics. However, the results may not be representative of the entire population.
Graphical Abstract
Highlights
- Small caliber vessel lesions tend to be very complicated, involving multiple vessels, distal locations, and limited margin of error in stent expansion, which is a challenge to current therapeutic approaches.
- Up to 16% of diabetic patients have an increased risk of restenosis and the requirement for revascularization and potential procedure-related complications.
- Ticagrelor has significant benefits compared to clopidogrel in reducing total death, stent thrombosis, and preventing cardiovascular events according to the PLATO (PLATelet inhibition and patient Outcomes) study but this effect was not well studied in type 2 diabetic patients with small diameter stents.
Introduction
Previous studies have indicated that factors such as diabetes duration, HbA1c levels, and type of antihyperglycemic treatment can impact cardiovascular events in patients with type 2 diabetes mellitus (T2DM).1,
The CHARISMA study and other cohort trials suggested that DAPT (Dual antiplatelets therapy) would offer protection above and beyond what aspirin offers.6-
Extra consideration needs to be given to diabetic patients after small diameter stents implantations, which accounts for 30%-70% of patients undergoing percutaneous coronary intervention (PCI)15 due to increased risk of restenosis and the requirement for revascularization and potential procedure-related complications.16 Small caliber vessel lesions tend to be very complicated, involving multiple vessels, distal locations, and type C lesion characteristics. The limited margin of error in stent expansion and sizing associated with small lumen size is a challenge to current therapeutic approaches. The aim of this study was to compare clinical outcomes between clopidogrel and ticagrelor in type 2 diabetic patients after implantation of small diameter stents at 1 year.
Study Population
Proactive, open-label, controlled registry trial in a single center. During the period of October 2021-November 2023, we included and monitored 332 patients with a history of coronary artery diseae and type 2 diabetes who were admitted to our hospital and were older than 18 but younger than 70 years. Our enrolled patients underwent PCI with implantation of small-diameter stents (SDS), that are equal to or less than 2.5 mm in diameter. Diabetes was defined as individuals with fasting blood glucose ≥126 mg/dL (7.0 mmol/L) or random blood glucose ≥200 mg/dL (11.1 mmol/L) or patients with a known history of ongoing hypoglycemic therapy. Pregnancy, any antiplatelet medication contraindications,17 the requirement for oral anticoagulation therapy, the concurrent use of strong cytochrome P450 3A inducers or inhibitors, the combination of chronic infections, malignant tumors, end-stage liver diseases, and life expectancy less than a year were the main exclusion criteria. The supplementary material provides information on the study’s inclusion and exclusion criteria.
Study Design
The study compared clinical outcomes between clopidogrel and ticagrelor in type 2 diabetic patients after implantation of SDS at 1 year. The choice of clopidogrel or ticagrelor, emergent or early invasive treatment strategies, stent type, pre-dilatation or post-dilatation, use of post-procedural glycoprotein IIb/IIIa inhibitors, and antithrombotic medication were established in accordance with the Patent’s clinical status and according to the clinical decision of operators under relevant guidelines and recommendations. A procedure was deemed successful if the target vessel had no residual stenosis following stenting, had a final TIMI flow grade of 3, and no major side branch occlusion or flow-limiting dissection following the procedure. Two groups of patients were identified: group 1 underwent DAPT with clopidogrel, and group 2 underwent DAPT with ticagrelor. The recommended loading and maintenance doses of aspirin, ticagrelor, and clopidogrel were given.10,
Artificial Intelligence Disclosure
We disclose that we did not use artificial intelligence (AI)-assisted technologies (such as Large Language Models [LLMs], chatbots, or image creators) in the production of this submitted work.
Follow-Up and Endpoints
Data have been collected about the patients' pre-existing conditions, medical background, risk factors, clinical diagnosis, medications used both upon admission and after discharge, results of in-hospital lab tests, and coronary procedures. Following PCI, each participant's general health condition, current medication, and end point-related events were recorded. This was done at follow-up appointments at the outpatient clinic or, if that was not feasible, over the phone at baseline and then again at 3, 6, 9, and 12 months.
The primary endpoint of the study was the occurrence of major adverse cardiovascular events (MACE) at 1 year, including composite (cardiac death, MI, stroke, and target vessel revascularization [TVR]), cardiac death, non-fatal MI, stroke, or TVR. As a secondary endpoint, the incidence of stent thrombosis, non-TVR, and bleeding (Bleeding Academic Research Consortium [BARC] 2, 3, or 5) was also examined. The fourth universal definition of MI was used to define it.19 Angiographic restenosis was defined as a percent diameter stenosis of 50% inside and just adjacent to the proximal and distal end of the stent. Target vessel revascularization was defined as repeat angioplasty or coronary bypass surgery performed because of restenosis for the target lesion or any segment of the artery containing the target lesion during follow-up. Death was classified as cardiac in origin unless clear non-cardiac causes could be identified.20,
Statistical Analysis
Continuous variables were expressed as mean ± SD or median and interquartile range, and categorical variables are represented by n (%). To check for normal distribution, either the Kolmogorov–Smirnov test was used.As appropriate, independent
Results
The study included 332 patients, of whom 197 patients were on clopidogrel and 135 on ticagrelor, as shown in the patient recruitment pathway in
It should be noted that left anterior descending coronary artery was the culprit vessel in 79 (23.7%) vs. 54 (16.2%) patients in the clopidogrel and ticagrelor groups, respectively. In the study, anterior wall MI accounted for 50.5% of all ST-segment myocardial infarction (STEMI) cases, with clopidogrel patients having higher rates of STEMI. The presentations and procedural outcomes among the clopidogrel and ticagrelor groups were comparable in
Analysis of Risk Factors of Outcomes
Logistic regression analysis of clinical characteristics, medical history, medications, laboratory biomarkers, and coronary angiography outcomes showed that male gender, history of ischemic heart disease, EF, coronary lesion type and presence of CKD were found to be potential predictors for the composite of CD, MI, stroke, TVR endpoint (
Clinical Outcomes and Survival Analysis
Univariate and multiple Cox proportional hazards regression were used to identify the factors affecting the survival outcome of primary and secondary endpoints, including the predictors from logistic regression analysis (
For the secondary endpoints, the total number of bleeding events defined by BARC 2, 3, or 5 did not reach significant statistical difference in crude analysis (HR = 1.315, 95% CI: 0.588-2.941,
Sub analysis of CKD patients, the 12-month rates of the composite of CD, MI, stroke, and TVR were lower in the ticagrelor group than in the clopidogrel group in propensity-matched patients (HR = 1.41, 95% CI: 0.619-3.558,
Discussion
Patients with DM have a higher incidence of cardiovascular events compared with nondiabetic patients, as a result of increased adhesion, chronic proinflammatory and prothrombotic environments that promote platelet activation.23,
While ticagrelor offers quicker and more favorable antiplatelet results, clopidogrel is the antithrombotic adjunct to aspirin that is most frequently administered.29,
Our study compared clinical outcomes between the clopidogrel and ticagrelor groups in patients with diabetes after stenting with small diameter stents. The rates of MACEs, bleeding, and net adverse clinical events did not significantly differ between individuals treated with ticagrelor or clopidogrel during a 12-month follow-up. These outcomes were comparable to those of earlier research projects including individuals with diabetes. According to research by Ahn et al, prasugrel/ticagrelor (n = 1000) significantly increased the number of major bleeding events compared to clopidogrel treatment (n = 2985) but did not improve the composite of cardiac mortality, recurrent MI, or stroke in MI patients with diabetes having PCI.33 In addition, Goto et al34 and Park et al35 discovered a minor rise in the number of major bleeding incidents in the ticagrelor treatment group. However, among Asian patients, there was no appreciable difference in the risk of ischemia between the ticagrelor and clopidogrel groups.33-
In the current study, male gender, history of ischemic heart disease, EF, coronary lesion type and presence of CKD were found to be potential predictors for the composite of CD, MI, stroke, TVR endpoint in univariate logistic regression model analysis. Further research is required on this. Without doubt, both DM and CKD are independently linked to a higher risk of cardiovascular ischemic events; moreover, these 2 conditions might compound the hazards when they co-exist.9,
The advantage of ticagrelor was seen in a subgroup analysis of the PLATO research, with patients with CKD (eGFR of <60 mL/min) seeing a higher absolute reduction in ischemia risk than those with normal renal function; however, the associated risk of bleeding increased with CKD stage.39 In our analysis, we found that 20%-25% of diabetic patients had CKD. This proportion is consistent with previous cardiovascular RCTs.8 Sub analysis of CKD patients in the present study demonstrated similar results at a 12-month follow-up, as rate of composite of CD, MI, stroke, and TVR was lower in the ticagrelor group than in the clopidogrel group in propensity-matched patients (HR = 1.41, 95% CI: 0.619-3.558,
Study Limitations
There are various constraints to consider when interpreting the results of this study. First, residual confounding, a known potential source of error in registry research, is made possible by the observational study design. Secondly, the absolute risk of bleeding is lower in the registry than the risk seen in clinical trials. It is conceivable that this relates to underreporting of bleeding events in the registry. However, this should impact the absolute number of bleeds and not the relative risk with ticagrelor. Third, intravascular imaging-guided stent deployment was not used in this study in demonstrating that coronary stenting is effective in establishing and maintaining small coronary artery patency without complications. Finally, a larger number of patients would increase the study’s power, resulting in more precise estimates. Further prospective multicenter trials enrolling larger patient numbers with diabetes, different risk factors, and ESRD are recommended to evaluate the outcomes in the Middle East population.
Conclusion
In conclusion, our study shows that ticagrelor did not improve the composite of cardiac death, MI, stroke, target vessel revascularization, or the risk of bleeding events defined by the BARC criteria in patients with diabetes and small diameter stents compared with clopidogrel emphasizing the importance of individualized treatment decisions based on patient characteristics and preferences. However, the results may not be representative of the entire population. Further research is warranted to validate these findings and explore long-term outcomes.
Footnotes
References
- Bajaj HS, Raz I, Mosenzon O. Cardiovascular and renal benefits of dapagliflozin in patients with short and long‐standing type 2 diabetes: analysis from the DECLARE‐TIMI 58 trial. Diabetes Obes Metab. 2020;22(7):1122-1131.
- Zelniker TA, Wiviott SD, Raz I. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022-2031.
- Stone JA, Houlden RL, Lin P, Udell JA, Verma S. Cardiovascular protection in people with diabetes. Can J Diabetes. 2018;42(suppl):S162-S169.
- LeRoith D, Biessels GJ, Braithwaite SS. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520-1574.
- Handelsman Y, Bloomgarden ZT, Grunberger G. American Association of Clinical Endocrinologists and American College of Endocrinology–Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015—executive summary. Endocr Pract. 2015;21(4):413-437.
- Bhatt DL, Fox KAA, Hacke W. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706-1717.
- Bhatt DL, Flather MD, Hacke W. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982-1988.
- James S, Angiolillo DJ, Cornel JH. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the platelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006-3016.
- Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123(7):798-813.
- Byrne RA, Rossello X, Coughlan JJ. ESC Guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44(38):3720-3826.
- Angiolillo DJ, Badimon JJ, Saucedo JF. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti-platelet Therapy in diabetes mellitus (OPTIMUS)-3 Trial. Eur Heart J. 2011;32(7):838-846.
- Bhatt DL, Bonaca MP, Bansilal S. . Reduction in Ischemic. 2016;54():-.
- Yang H, Tang B, Xu CH, Ahmed A. Ticagrelor versus prasugrel for the treatment of patients with type 2 diabetes mellitus following percutaneous coronary intervention: a systematic review and meta-analysis. Diabetes Ther. 2019;10(1):81-93.
- Franchi F, Rollini F, Aggarwal N. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the Optimus (optimizing antiplatelet therapy in diabetes mellitus)-4 study. Circulation. 2016;134(11):780-792.
- Colombo A, Chieffo A. Drug-eluting stent update 2007: Part III: Technique and unapproved/unsettled indications (left main, bifurcations, chronic total occlusions, small vessels and long lesions, saphenous vein grafts, acute myocardial infarctions, and multivessel disease). Circulation. 2007;116(12):1424-1432.
- Cassese S, Byrne RA, Tada T. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153-159.
- Yun KH, Cho JY, Lee SY. Ischemic and bleeding events of ticagrelor monotherapy in Korean patients with and without diabetes mellitus: insights from the TICO trial. Front Pharmacol. 2020;11():620906-.
- Knuuti J, Wijns W, Saraste A. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477.
- Thygesen K, Alpert JS, Jaffe AS. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-e651.
- Cutlip DE, Windecker S, Mehran R. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
- Garcia-Garcia HM, McFadden EP, Farb A. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018;137(24):2635-2650.
- Mehran R, Rao SV, Bhatt DL. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747.
- Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015;120():28-39.
- Jung JH, Tantry US, Gurbel PA, Jeong YH. Current antiplatelet treatment strategy in patients with diabetes mellitus. Diabetes Metab J. 2015;39(2):95-113.
- Price MJ, Saito S, Shlofmitz RA. First report of the Resolute Onyx 2.0-mm zotarolimus-eluting stent for the treatment of coronary lesions with very small reference vessel diameter. JACC Cardiovasc Interv. 2017;10(14):1381-1388.
- Siontis GCM, Piccolo R, Praz F. Percutaneous coronary interventions for the treatment of stenoses in small coronary arteries: a network meta-analysis. JACC Cardiovasc Interv. 2016;9(13):1324-1334.
- Sim HW, Ananthakrishna R, Chan SP. Treatment of very small de novo coronary artery disease with 2.0 mm drug-coated balloons showed 1-year clinical outcome comparable with 2.0 mm drug-eluting stents. J Invasive Cardiol. 2018;30(7):256-261.
- Jen HL, Wang YC, Tsao TP, Yin WH. Percutaneous Coronary Intervention for Very Small Vessels with the Use of a Newer-Generation 2.0 mm Drug-Eluting Stent. J Invasive Cardiol. 2021;33(7):E565-E574.
- Mangiacapra F, Panaioli E, Colaiori I. Clopidogrel versus ticagrelor for antiplatelet maintenance in diabetic patients treated with percutaneous coronary intervention: results of the clotildia study (clopidogrel High Dose versus ticagrelor for antiplatelet Maintenance in Diabetic Patients). Circulation. 2016;134(11):835-837.
- Sweeny JM, Angiolillo DJ, Franchi F. Impact of diabetes mellitus on the pharmacodynamic effects of ticagrelor versus clopidogrel in troponin‐negative acute coronary syndrome patients undergoing ad hoc percutaneous coronary intervention. J Am Heart Assoc. 2017;6(4):e005650-.
- Angiolillo DJ, Jakubowski JA, Ferreiro JL. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64(10):1005-1014.
- Marcano AL, Gracida M, Roura G. Antiplatelet efficacy of ticagrelor versus clopidogrel in Mediterranean patients with diabetes mellitus and chronic coronary syndromes: a crossover pharmacodynamic investigation. Front Cardiovasc Med. 2022;9():1057331-.
- Ahn KT, Seong SW, Choi UL. Comparison of 1-year clinical outcomes between prasugrel and ticagrelor versus clopidogrel in type 2 diabetes patients with acute myocardial infarction underwent successful percutaneous coronary intervention. Medicine (United States). 2019;98(11):e14833-.
- Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome–randomized, double-blind, phase III PHILO study–. Circ J. 2015;79(11):2452-2460.
- Park DW, Kwon O, Jang JS. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140(23):1865-1877.
- Capodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125(21):2649-2661.
- James S, Budaj A, Aylward P. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the platelet inhibition and patient outcomes (Plato) trial. Circulation. 2010;122(11):1056-1067.
- Franchi F, James SK, Ghukasyan Lakic T. Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y12 receptor antagonist effects in patients with acute coronary syndromes: insights from the Plato trial. J Am Heart Assoc. 2019;8(6):e011139-.
- Gao C, Tomaniak M, Takahashi K. Ticagrelor monotherapy in patients with concomitant diabetes mellitus and chronic kidney disease: a post hoc analysis of the global leaders trial. Cardiovasc Diabetol. 2020;19(1):179-.
- Edfors R, Sahlén A, Szummer K. Outcomes in patients treated with ticagrelor versus clopidogrel after acute myocardial infarction stratified by renal function. Heart. 2018;104(19):1575-1582.


