Abstract
Background: The objective is to compare the frequency and clinical characteristics of pediatric Postural Orthostatic Tachycardia syndrome (POTS) diagnoses before and during the Coronavirus Disease 2019 (COVID-19) pandemic and assess potential contributing factors.
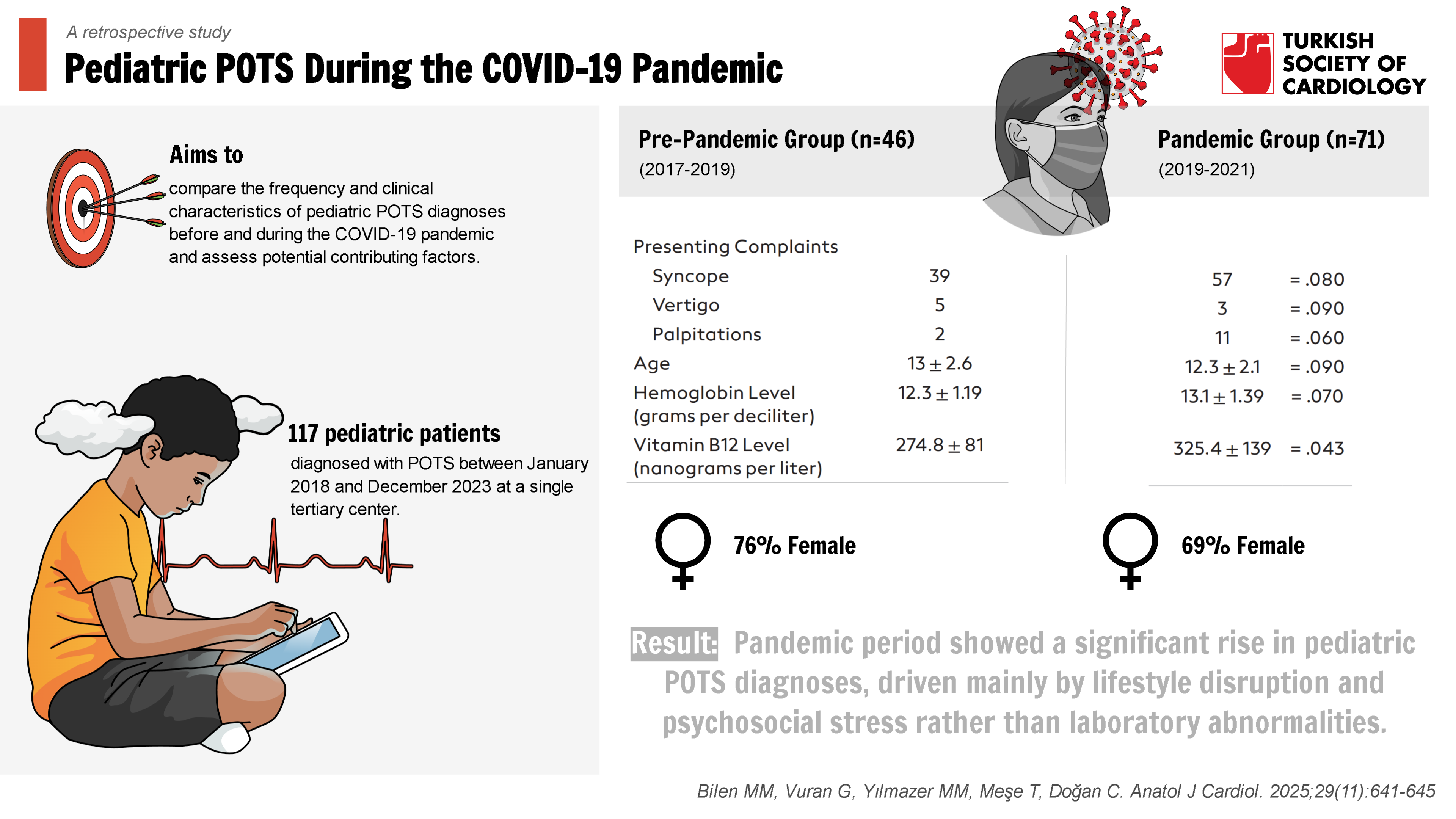
Methods: This retrospective study analyzed 117 pediatric patients diagnosed with POTS between January 2018 and December 2023 at a single tertiary center. Patients were
divided into pre-pandemic (n = 46) and pandemic (n = 71) groups. Clinical, laboratory, and psychosocial data were collected and compared. Logistic regression was used to identify independent predictors of pandemic-period diagnoses.
Results: A significant increase in POTS diagnoses was observed during the pandemic (10.5% vs. 6.1%, P= .01). Pandemic-period patients reported lower physical activity (72%) and higher screen time (85%), with increased symptoms of anxiety (34% vs. 18%, P= .04) and palpitations (P= .03). Vitamin B12 levels were higher in the pandemic group (P= .043), while hemoglobin levels and heart rate variability remained similar across groups.
Conclusion: The COVID-19 pandemic was associated with a marked rise in pediatric POTS diagnoses, likely driven by lifestyle alterations and psychosocial stress rather than nutritional deficiencies. These findings underscore the importance of early recognition, physical reconditioning, and psychological support in managing POTS, particularly during global health crises.
Graphical Abstract
Highlights
- The study cohort consisted of patients referred to a tertiary pediatric cardiology center between January 2019 and December 2023. This single-center population may not fully represent the general pediatric population.
- This study demonstrates an increase in pediatric POTS diagnoses during the COVID-19 pandemic, likely related to increased viral exposure, reduced physical activity, and elevated psychological stress.
- The primary contributing factors to POTS appear to be external stressors rather than nutritional deficiencies.
- Lifestyle changes such as reduced exercise and increased screen time, along with psychological stress, emerged as key contributors to the exacerbation of POTS symptoms.
Introduction
Postural Orthostatic Tachycardia syndrome (POTS) is characterized by an excessive increase in heart rate upon standing and is commonly associated with symptoms such as dizziness, palpitations, and syncope.1 Postural Orthostatic Tachycardia Syndrome is particularly prevalent during childhood and adolescence, and its pathophysiology involves a complex interplay of autonomic dysfunction, immune system factors, and physical deconditioning.2 The COVID-19 pandemic brought about unprecedented changes in daily life, including lockdowns, social isolation, and increased psychological stress.3 These factors are believed to potentially influence the incidence and clinical presentation of POTS.
Due to the pandemic, there were major alterations in routine activities: children spent more time indoors, participated in remote learning, and experienced limited opportunities for physical activity. These lifestyle changes, combined with the stress and uncertainty associated with the pandemic—particularly the reduction in physical activity and increased psychosocial burden—may have contributed to a rise in POTS cases.4 Moreover, increased exposure to viral infections, including severe acute respiratory syndrome coronavirus 2, may have triggered autoimmune mechanisms that further disrupt autonomic regulation.5 Understanding the impact of these factors on the diagnosis and clinical course of POTS is crucial for improving patient outcomes.
This study aims to compare the frequency and characteristics of pediatric POTS diagnoses before and during the COVID-19 pandemic. It is hypothesized that pandemic-related factors—such as increased viral infections, reduced physical activity, and heightened stress—have contributed to a rise in POTS incidence and altered its clinical presentation. Understanding these changes may aid in the development of future management strategies during similar crises and improve patient care.
Methods
All legal guardian consents were obtained, and verbal assent was received from patients aged 15 years and older.
Inclusion criteria were based on established diagnostic criteria for POTS, including an increase in heart rate of ≥40 beats per minute upon standing or during a head-up tilt test, along with the presence of orthostatic intolerance symptoms for at least 3 months.
Patients diagnosed with conditions that could account for symptoms unrelated to POTS—such as cardiac arrhythmias of non-POTS origin, structural heart diseases, or severe anemia secondary to thalassemia major or hereditary hemoglobinopathies—were excluded from the study.
The distinction between the pre-pandemic and post-pandemic periods was defined based on the timeline when the national Ministry of Health policies regarding COVID-19 and associated social restrictions were largely lifted, and clinical practices at the study center were subsequently revised accordingly.
All patients included in the evaluation and subjected to the tilt table test were initially assessed by pediatric specialists at the authors’ center, and relevant blood tests were conducted to investigate potential causes of their symptoms. Therefore, data on vitamin B12 levels and hemoglobin values were available for all patients.
Demographic data, clinical symptoms, laboratory findings (including serum vitamin B12 and hemoglobin levels), and diagnostic information were collected from electronic health records. Symptoms such as syncope, dizziness, palpitations, and fatigue were documented. Data on physical activity levels, screen time, and psychosocial stress factors during the pandemic were obtained through interviews with patients and caregivers when available. Due to the retrospective nature of the study, standardized anxiety assessment scales could not be utilized. Screen time was based on patient or caregiver self-report and was not measured using a standardized tool.
During the pandemic period, patients’ SARS-CoV-2 polymerase chain reaction results could not be retrieved from medical records, as this data were restricted under the Ministry of Health’s patient privacy regulations. Consequently, it was not possible to directly assess the association between the findings and confirmed COVID-19 infection.
Ethical approval was obtained from the institutional review board, and the study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Statistical analyses were performed using SPSS version 26. Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as mean ± SD. Comparisons between the pre-pandemic and pandemic groups were conducted using the chi-square test for categorical variables and the independent samples
Although logistic regression analysis can be useful in identifying independent factors associated with the diagnosis of POTS during the pandemic period, the authors did not conduct such an analysis in their study due to the small sample size and its retrospective design.
Results
Out of the 750 head-up tilt tests performed before the pandemic, 46 (6.1%) resulted in a diagnosis of POTS, while 71 (10.5%) out of the 670 tests conducted during the pandemic were diagnosed with POTS. When comparing the 2 periods statistically, the diagnosis rate during the pandemic was significantly higher (
Before the pandemic, 84% (n = 39) of the presenting complaints were syncope, 10% (n = 5) were vertigo, and 6% (n = 2) were palpitations. During the pandemic, 80% (n = 57) of the complaints were syncope, 4.2% (n = 3) were vertigo, and 15.8% (n = 11) were palpitations. When comparing the presenting complaints between the 2 periods, no statistically significant difference was found.
Before the pandemic, the patients’ average hemoglobin level was 12.3 ± 1.19 g/dL, while during the pandemic, it was 13.1 ± 1.39 g/dL. When examining hemoglobin levels, no statistically significant difference was found between the pre-pandemic and pandemic periods (
Before the pandemic, the average vitamin B12 level of the patients was 274.8 ± 81 ng/L, while during the pandemic, it was 325.4 ± 139 ng/L. When comparing vitamin B12 levels between the 2 periods, the levels were statistically higher during the pandemic (
Electrocardiographic findings in both periods showed no clinically significant results, so no comparison between the 2 periods was made in this regard.
Discussion
Our study demonstrates a significant increase in the diagnosis rate of POTS during the COVID-19 pandemic. This rise is likely attributable to a range of factors including increased exposure to SARS-CoV-2, a sedentary lifestyle, and heightened psychological stress. Increased viral exposure may have triggered autoimmune mechanisms that contribute to the pathogenesis of POTS. Autoantibodies targeting adrenergic and muscarinic receptors are known to play a role in POTS, and viral infections have been shown to stimulate the production of such autoantibodies.6-
In this study, syncope was found to be the most common presenting complaint. This is attributable to the fact that the authors’ center is a tertiary care institution. Due to the availability of pediatric neurology, pediatric cardiology, and advanced central imaging modalities, patients requiring further investigation and evaluation—such as those presenting with syncope—are frequently referred to the authors’ center. This has contributed to a shift in the typical pattern of patient presentations.
The pandemic also brought about notable lifestyle changes such as reduced physical activity and increased screen time. Reduced physical activity is a known risk factor that impairs cardiovascular responses to orthostatic stress, thereby exacerbating POTS symptoms.10-
Unexpectedly higher serum vitamin B12 levels in the pandemic group may reflect increased use of multivitamin supplements aimed at supporting immune function during this period. While vitamin B12 is critical for maintaining autonomic function and its deficiency is linked to autonomic dysfunction,18-
Consistent with previous literature,23 this study found that POTS was more prevalent among female patients, accounting for approximately 70-76% of cases. This gender disparity may be related to the higher incidence of autoimmune conditions and hormonal differences in adolescent females.24 These findings underscore the importance of considering gender-specific factors in the diagnosis and management of pediatric POTS.
The predominance of female patients in both groups aligns with existing evidence that adolescent girls are more vulnerable to POTS due to hormonal fluctuations and increased rates of autoimmune disease. The interaction between estrogen and the autonomic nervous system may partly explain this increased susceptibility.25 Additionally, adolescent girls’ heightened exposure to social stressors may exacerbate autonomic symptoms and elevate POTS risk.
These findings highlight the need for increased awareness of POTS during periods of heightened viral exposure and lifestyle disruption. Non-pharmacologic interventions—such as increasing fluid and salt intake, maintaining physical activity, and providing psychological support—are essential. Given the role of anxiety in exacerbating POTS symptoms, mental health support is particularly important during times of elevated stress. Personalized interventions that address both the physiological and psychological components of POTS may improve patient outcomes. Enhanced collaboration among cardiologists, neurologists, and child psychiatrists could foster a more holistic approach to care.
Early intervention is key. Encouraging physical activity, including light exercise, may enhance autonomic function and reduce symptom burden. Cognitive-behavioral therapy (CBT) or stress management techniques may help alleviate autonomic hyperactivity associated with POTS and improve quality of life.
Study Limitations
This study has several limitations, including its retrospective and single-center design, as well as a relatively small sample size, all of which may limit the generalizability of the findings. Furthermore, long-term follow-up data are lacking, preventing assessment of the chronic impact of pandemic-related lifestyle changes on POTS. The potential influence of COVID-19 vaccination on POTS incidence could not be evaluated, which represents an important area for future research. Although the authors observed an increased number of POTS diagnoses following the pandemic onset, their findings should be interpreted with caution due to the limited and single-center nature of the sample. Population-based studies are needed to confirm the true incidence and prevalence of pediatric POTS in the post-pandemic era. Larger, multi-center, longitudinal studies are needed to validate these findings, assess the potential impact of vaccination, and provide deeper insights into pediatric POTS management.
The absence of detailed pre-pandemic lifestyle data (e.g. exercise frequency and screen time) limits the ability to precisely quantify their effects on POTS incidence. Future prospective studies should aim to collect more comprehensive data on lifestyle factors.
Conclusion
The COVID-19 pandemic has significantly influenced the incidence and clinical features of pediatric POTS. Increased viral exposure, reduced physical activity, and elevated psychological stress likely contributed to the rise in POTS diagnoses during the pandemic. Early diagnosis and appropriate management are essential to mitigate the impact of POTS, especially during global health crises. Emphasizing the importance of addressing both physical and psychological dimensions of POTS is critical—particularly during periods of heightened external stress. Future research should focus on the long-term effects of these factors, the role of vaccinations, and targeted management strategies.
Lessons learned from the pandemic may inform future healthcare strategies for managing autonomic dysfunctions like POTS in the context of similar global health events. Proactive identification of at-risk populations, early intervention, and a multidisciplinary approach will be key to improving outcomes for pediatric patients facing autonomic challenges during times of crisis.
Footnotes
References
- Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127(23):2336-2342.
- Stewart JM, Weldon A. Vascular perturbations in the chronic orthostatic intolerance of the postural orthostatic tachycardia syndrome. J Appl Physiol (1985). 2000;89(4):1505-1512.
- Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18(4):508-509.
- Dani M, Dirksen A, Taraborrelli P. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63-e67.
- González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA. Post-acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a Mexican cohort. Brain Sci. 2021;11(6):760-.
- Watari M, Nakane S, Mukaino A. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. 2018;5(4):486-492.
- Li H, Yu X, Liles C. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3(1):e000755-.
- Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor antibodies. J Am Heart Assoc. 2019;8(18):e013602-.
- Yu X, Li H, Murphy TA. Angiotensin II Type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc. 2018;7(8):e008351-.
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205-211.
- Shouman K, Vanichkachorn G, Cheshire WP. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385-394.
- Guilmot A, Maldonado Slootjes S, Sellimi A. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268(3):751-757.
- Wallukat G, Hohberger B, Wenzel K. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4():100100-.
- Theoharides TC. Could SARS-CoV-2 spike protein be responsible for longCOVID syndrome?. Mol Neurobiol. 2022;59():1850-1861.
- Hugon J, Msika E-F, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44-46.
- Loughnan A, Gall N, James S. Observational case series describing features of cardiopulmonary exercise testing in postural tachycardia syndrome (PoTS). Auton Neurosci. 2021;231():102762-.
- Motiejunaite J, Balagny P, Arnoult F. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors?. Front Physiol. 2020;11():614590-.
- Toru S, Yokota T, Inaba A. Autonomic dysfunction and orthostatic hypotention caused by vitamin B12 deficiency. J Neurol Neurosurg Psychiatry. 1999;66(6):804-805.
- Sanya EO, Tutaj M, Brown CM, Goel N, Neundörfer B, Hilz MJ. Abnormal heart rate and blood pressure responses to baroreflex stimulation in multiple sclerosis patients. Clin Auton Res. 2005;15(3):213-218.
- Gao MZ, Li HQ. Vitamin B12 nutritional status in preschool children in Chongqing. Zhonghua Er Ke Za Zhi. 2006;44(1):7-10.
- Rosenbaum DH, Cain MP, Kaefer M. Ileal enterocystoplasty and B12 deficiency in pediatric patients. J Urol. 2008;179(4):1544-1548.
- Yağci M, Yamaç K, Acar K, Cingi E, Kitapçi M, Haznedar R. Gastric emptying in patients with vitamin B(12) deficiency. Eur J Nucl Med Mol Imaging. 2002;29(9):1125-1127.
- Thieben MJ, Sandroni P, Sletten DM. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308-313.
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286(1):H449-H457.
- Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci. 2018;215():78-82.


