2Department of Cardiology, Pamukkale University, Denizli, Türkiye
3Department of Cardiology, Isparta City Hospital, Isparta, Türkiye
4Department of Cardiology, Denizli State Hospital, Denizli, Türkiye
5Department of Infection Diseases and Clinical Microbiology, Pamukkale University, Denizli, Türkiye
Abstract
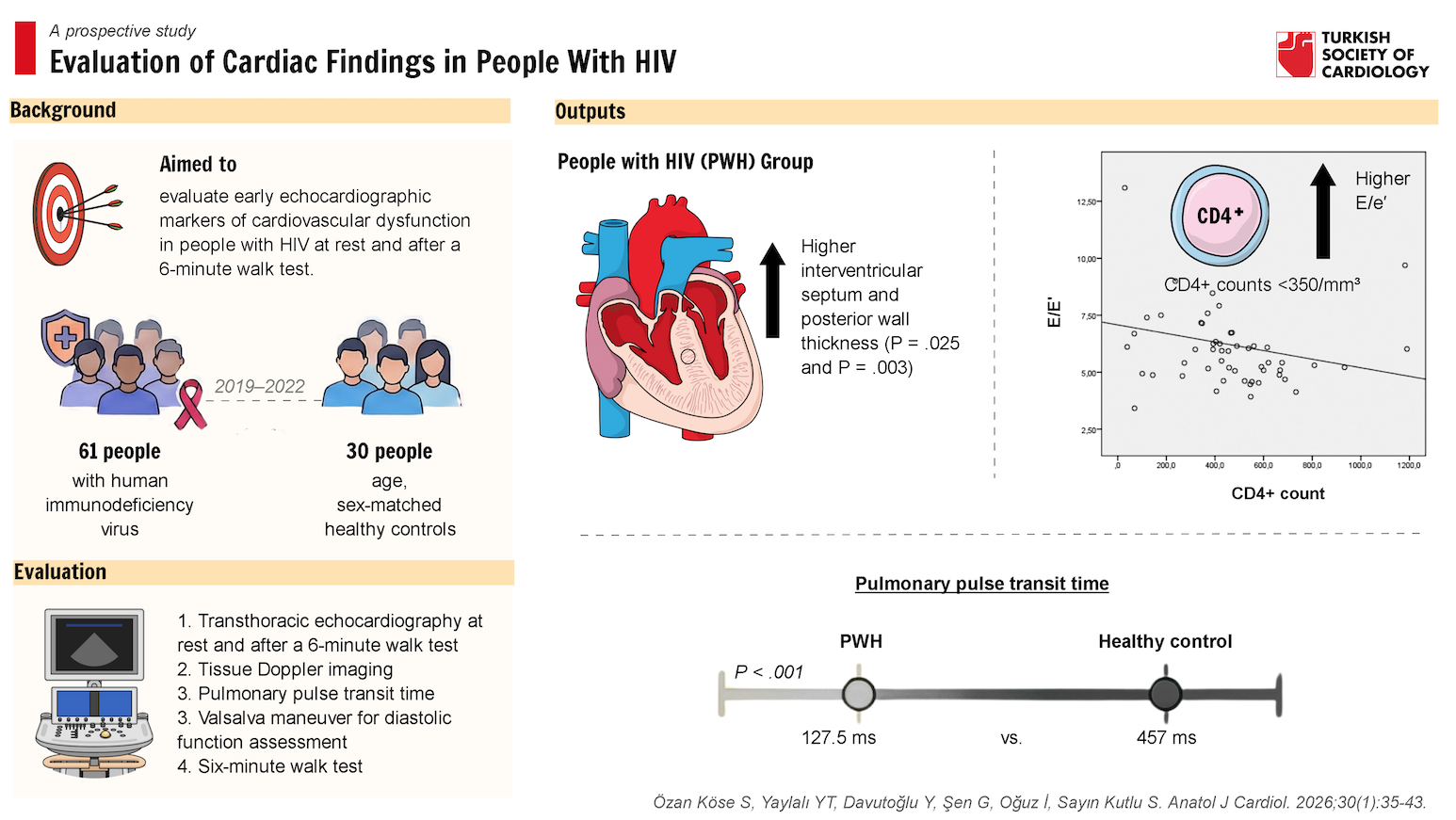
Objective: Although opportunistic infections and malignancies have declined due to antiretroviral therapy, the prevalence of cardiovascular disease (CVD) among people with human immunodeficiency virus (PWH) has increased. This study examines early markers of CVD using transthoracic echocardiography (TTE) performed at rest and after a 6-minute walk test (6-MWT) in PWH. This prospective study was conducted in Türkiye between 2019 and 2022.
Methods: The PWH and healthy individuals were evaluated for demographic and laboratory analysis and examined using TTE at rest and after 6-MWT.
Results: The interventricular septum (IVS) and posterior wall (PW) were significantly thicker in the PWH group than in healthy controls. Pulmonary pulse transit time (pPTT)
was markedly reduced in PWH (127.5 ms vs. 457 ms, P < .001). In the PWH group, the E/e’ ratio increased after 6-MWT [6.26 (IQR: 5.1-7.3) vs. 5.9 (IQR: 4.9-6.9) at rest (P = .028)]. The PWH with CD4+ counts <350/mm3 exhibited a higher E/e’ ratio [6.91 (IQR: 5.05-8.62)] than those with CD4+ counts >350/mm3 [5.41 (IQR: 4.87-6.17); P = .035]. A weak inverse correlation was observed between CD4+ count and E/e’ ratio (P = .010, r = −0.348).
Conclusions: The IVS thickness, PW thickness, E/e’ ratio, and pPTT may serve as valuable parameters for the early detection of CVD in PWH. Changes in diastolic indices may offer insights into disease progression. The pPTT may be a promising marker for evaluating the pulmonary vascular status and right ventricular function. These findings underscore the need for further research into the diagnostic and prognostic utility of diastolic parameters and pPTT in the clinical management of PWH.
Graphical Abstract
Highlights
- Identifying predictors of cardiovascular disease in people with human immunodeficiency virus (PWH) is essential for early intervention and improved outcomes.
- Reduced pulmonary pulse transit time may reflect early vascular alterations in the pulmonary circulation among PWH.
- The E/e’ ratio, a marker of left ventricular (LV) end-diastolic pressure may be valuable for monitoring diastolic function, particularly in PWH with CD4+ count below 350.
- Structural markers of LV deterioration, such as interventricular septum and posterior wall thickness, may aid in the longitudinal assessment of cardiac health in PWH.
Introduction
Human immunodeficiency virus (HIV) causes immunosuppression and leads to the development of opportunistic infections and cancers.1
Highly active antiretroviral therapy (HAART) has been shown to reduce deaths from these complications in people with HIV (PWH) in recent years. The PWH are now facing an increasing burden of chronic non-infectious conditions, particularly cardiovascular diseases (CVD).2-
Subclinical cardiac dysfunction may precede clinical symptoms, suggesting that early detection could be beneficial in this population. Inflammation and immune activation persist to some level, even in patients who have achieved viral suppression. Prolonged inflammation increases the risk of morbidity and mortality by inducing endothelial dysfunction, vascular remodeling, and myocardial alterations, which contribute to conditions such as diastolic dysfunction (DD) and pulmonary hypertension (PH). Plexiform lesions, along with cellular proliferation and hypertrophy, are observed across the intima, media, and adventitia. The HIV-associated PH is believed to result from the combined effects of HIV viral proteins and proinflammatory cytokines.5-
Methods
This prospective study was conducted at Pamukkale University Hospital, Departments of Infectious Diseases and Clinical Microbiology and Cardiology. Between January 2019 and December 2022, all patients who met the inclusion criteria and had no exclusion criteria were consecutively enrolled in the study. A post hoc power analysis was performed. The control group consisted of HIV-negative individuals with no comorbidities. The PWH and control groups were matched for age and sex in a 2:1 ratio. All study participants were evaluated for demographic and laboratory analysis and examined with TTE at rest and after 6-MWT. A total of 61 PWH and 30 healthy individuals were included in the study.
Exclusion criteria included ejection fraction (EF) <50%, congenital heart disease, connective tissue disease, PH, chronic obstructive pulmonary disease, acute or chronic renal failure, pregnancy, DD grade 2 or higher, obstructive sleep apnea syndrome, malignancy, valvular heart disease, chronic thromboembolic PH, vasculitis, hematological disease, metabolic storage disease, coronary artery disease, and inadequate echocardiographic imaging. Echocardiographic measurements in PWH were compared at rest and after 6-MWT.
Artificial intelligence-supported technologies (e.g., large language models, chatbots, or image generators) were not used in this study.
Transthoracic Echocardiography
Philips® Affiniti 50C Vascular Ultrasound System and Philips® S5-1 ultrasound probe were used for the measurements.
All echocardiographic results were analyzed by a cardiologist blinded to the patient’s clinical and laboratory characteristics. Intraobserver variability of <5% was accepted for the echocardiographic measurements. Measurements were repeated 1 week apart. Only 1 reader conducted this analysis. The reader was allowed to select the best measurement each time and was blinded to previous measurements. Intraobserver variability was calculated as the difference between 2 measurements of the same patient by a single cardiologist divided by the mean value.
An echocardiographic evaluation was performed after the participants rested for at least 10 minutes, in the left lateral recumbent position. After continuous single-lead electrocardiography (ECG) monitoring, standard 2-dimensional, M-mode, and color Doppler evaluations were performed. The mitral valve flow velocities were measured using the pulse wave doppler (PWD) technique after the probes were positioned at the measurement region of the mitral valve tips from the apical 4-chamber view. Peak early (E) and late (A) diastolic mitral inflow velocities were obtained. Peak early (E’) and late (A’) diastolic myocardial velocities were measured. Measurements were obtained from both the lateral and septal mitral annuli, and the average of these values was used for analysis.15
In a healthy heart during the Valsalva maneuver, early diastolic filling velocity (E wave) decreases, while atrial contraction velocity (A wave) remains relatively unchanged or slightly increases. Thus, the E/A ratio decreases mildly. However, in latent (hidden) DD, the E wave decreases markedly with Valsalva, and the A wave either remains the same or becomes relatively higher. The E/A ratio drops significantly, often falling below 1 post-Valsalva. The diastolic filling pattern shifts from a pseudonormal (appearing normal at rest) to an abnormal relaxation pattern. Thus, a significant drop in the E/A ratio during Valsalva suggests latent DD, which means DD is masked at rest but revealed under reduced preload conditions.15 The Valsalva maneuver was used to unmask latent DD. The 6-MWT was performed to assess functional capacity. It was conducted in a 30-meter hallway within the cardiology outpatient clinic, using a stopwatch and pulse oximeter for timing and monitoring.16,
After identifying the location of the flow using color Doppler, continuous-wave Doppler was applied at the level of the tricuspid valve tips to measure tricuspid regurgitation velocity (TRV). Systolic pulmonary artery pressure (sPAP) was estimated using the simplified Bernoulli equation by multiplying 4 by the square of the peak TRV and adding the estimated right atrial pressure (RAP). The RAP was estimated noninvasively based on inferior vena cava (IVC) size and its respiratory variation.
sPAP = 4 × (TRVmax2) + RAP18,
The left atrial volume was calculated using the left atrial area (A1) obtained at the end of left ventricular (LV) systole, when the left atrium is at its largest, the left atrial area (A2) from the apical 2-chamber view, and the vertical length (L) of the left atrium.
Left atrial volume index (LAVI) was measured using the biplane area-length method based on images obtained from both the apical 4-chamber (A4C) and apical 2-chamber (A2C) views.20,
Pulmonary pulse transit time was measured by determining the time interval between the peak of the R-wave in the ECG and the peak flow velocity of the pulmonary vein during late systole. The LV EF was obtained using the modified Simpson’s method. In the parasternal short-axis view, pulmonary peak velocity was measured with PWD, and the pulmonary flow acceleration time (PAT), defined as the time from pulmonary valve opening to the systolic peak, was also obtained.
Pulmonary arterial stiffness (PAS) was calculated as the ratio of the maximum frequency shift (MFS), measured in hertz (Hz), to the PAT, measured in milliseconds (ms).
PAS = MFS (Hz)/PAT (ms)22
Statistical Analysis
The data were analyzed using IBM SPSS Statistics version 22.0. Normality was assessed with the Shapiro–Wilk test. Variables with normal distribution were evaluated using the Student’s
To investigate the association between pPTT and various demographic, clinical, laboratory, and echocardiographic parameters, univariate and multiple models (multiple linear regression) were conducted, with pPTT as the dependent variable. Two multiple models were constructed. Model 2 included variables obtained after 6-MWT. Variables significant in univariate analysis were included in the multiple models.
Results
Sixty-one PWH and 30 age- and sex-matched healthy subjects were enrolled in this study. Both groups were similar in terms of demographic and clinical parameters. However, the prevalence of overweight individuals [body mass index (BMI) = 25-29.9] was significantly higher in the control group [13 (22.8%),
Five PWH were receiving protease inhibitor therapy: 2 had transitioned from integrase inhibitors, 2 had used protease inhibitors consistently, and 1 had switched to integrase inhibitors. Echocardiographic findings are presented in
A statistically significant but weak negative correlation was found in PWH between CD4+ count at diagnosis and the E/e′ ratio (
Mitral E velocity increased from 74.8 ± 13.3 cm/s at rest to 84.6 ± 16.7 cm/s after 6-MWT. Mitral A velocity rose from 58 cm/s (IQR: 50-74 cm/s) to 66 cm/s (IQR: 58.5-82 cm/s), and e’ velocity increased from 13.5 cm/s (IQR: 10.5-15.5 cm/s) to 14 cm/s (IQR: 11.5-16.5 cm/s). All changes were statistically significant (
Among PWH, although E/e’ ratio values remained within normal limits, they increased significantly from 5.9 (IQR: 4.9-6.9) at rest to 6.26 (IQR: 5.1-7.3) after 6-MWT (
Tricuspid E velocity increased from 54.8 ± 11.5 cm/s at rest to 58.5 ± 13.4 cm/s after 6-MWT (
In 2 separate univariate analyses, CD4+ count at initial diagnosis, total cholesterol, mitral E to A ratio post-Valsalva maneuver, and sPAP were identified as significant predictors of pPTT. Additionally, mitral E velocity after 6-MWT and post-Valsalva maneuver, TRV after 6-MWT, IVC diameter after 6-MWT, and sPAP after 6-MWT were also significantly associated with pPTT. Univariate analysis revealed no significant association between pPTT and obesity, overweight status, smoking, or alcohol consumption. In the multiple analysis, CD4+ count at diagnosis, sPAP, mitral E/A ratio post-Valsalva maneuver, and TRV after 6-MWT remained significant predictors of pPTT (
Discussion
Cardiac findings were assessed via TTE at rest and after 6-MWT in PWH. The primary findings included a weak inverse correlation between CD4+ count at diagnosis and the E/e’ ratio, modest increases in IVS and PW thickness, reductions in E velocity and pPTT, and a statistically significant yet clinically modest after 6-MWT increase in the E/e’ ratio. Importantly, no definitive evidence of PH or overt DD was observed. Although individuals with CD4+ counts <350/mm3 exhibited slightly higher E/e′ ratios, these values remained within the normal reference range. In the multiple analysis, pPTT remained significantly associated with CD4+ count at diagnosis, sPAP, mitral E/A ratio post-Valsalva maneuver, and TRV after 6-MWT. No significant structural or diastolic functional differences were identified between users of new-generation integrase inhibitors or between users and non-users of protease inhibitors, despite their established associations with HIV-related CVD and hyperlipidemia.23 This may reflect the relatively young and otherwise healthy profile of the study cohort, except for smoking.
Obesity is a well-established risk factor for cardiovascular mortality. Previous studies have demonstrated that increased BMI and systolic blood pressure (SBP) are associated with greater IVS and PW thickness.24 However, in this study, BMI and SBP were comparable between groups, and while IVS and PW thicknesses were higher in PWH, the differences were minor. These subtle structural changes may not represent early pathological hypertrophy, and their clinical relevance remains uncertain. Furthermore, no significant associations were observed between reduced pPTT and obesity, overweight status, smoking, or alcohol consumption among PWH.
Longitudinal studies are warranted to better understand the clinical relevance of the echocardiographic changes observed in this population. Although LV hypertrophy (LVH) has been associated with chronic inflammatory conditions such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, the applicability of these findings to PWH remains speculative.25,
An elevated E/e’ ratio has been associated with increased CVD risk and mortality and may serve as an early marker for heart failure with a preserved EF (HFpEF). In this study, HIV-infected individuals with CD4+ counts <350/mm3 exhibited slightly higher E/e’ ratios compared to those with CD4+ counts >350/mm3; however, these values remained within the normal reference range. A weak inverse correlation between CD4+ count and E/e’ ratio was observed, suggesting a potential relationship between immune status and diastolic function. While lower CD4+ T lymphocyte levels have been linked to chronic systemic inflammation,34 which may contribute to cardiovascular alterations, the clinical significance of the observed E/e’ differences in the asymptomatic and relatively young population remains uncertain. Moreover, the lack of association between C-reactive protein (CRP) levels and reduced pPTT in the study further underscores the need for cautious interpretation of inflammatory markers and their relationship with subclinical echocardiographic changes.
A study from West Africa reported an association between reduced LV function and severe DD in PWH with CD4+ T lymphocyte counts below 500 cells/mm3.35 However, that study involved participants with more advanced diastolic abnormalities than those included in the cohort. In contrast, the study population consisted exclusively of asymptomatic, relatively young individuals without major cardiovascular risk factors, and participants with DD of grade 2 or higher were excluded. The subtle echocardiographic changes observed, such as borderline variations in diastolic parameters, may reflect early functional alterations potentially linked to HIV infection, although these findings must be interpreted with caution. Given that HIV was well controlled in the participants, the extent to which these subclinical changes are attributable to viral effects versus incidental variation remains unclear. Previous studies, such as that by Hsue et al,36 have suggested a potential link between HIV infection and LVH or DD, but the findings do not provide definitive support for such associations and should be considered preliminary.
In the study by Hsue et al,36 50% of asymptomatic HIV-positive patients demonstrated evidence of mild DD on TTE, compared to 29% of control subjects. Notably, the E/e’ ratio was higher in individuals with CD4+ counts below 350/mm3, suggesting that immune dysfunction may contribute to elevated E/e’ values. However, this relationship remains associative and does not establish causality. In contrast, Mondy et al37 reported a DD prevalence of 26%; however, their analysis found no significant association between the presence of DD or LVH on TTE and markers of HIV disease status, such as elevated viremia, CD4+ nadir, or CD4+ count at the time of echocardiography. These contrasting findings highlight the heterogeneity across study populations and methodologies. The ongoing
Previous studies have reported DD and LVH as among the most commonly observed echocardiographic abnormalities in PWH.39-
In diagnosing PH, pPTT, which measures the interval from the onset of the pulmonary artery pulse wave to its arrival in the left atrium, is being explored as a novel parameter. First demonstrated by Wibmer et al,42 pPTT was found to be significantly shorter in patients with SLE and PH compared to controls. Furthermore, a shorter pPTT duration was associated with increased vascular wall stiffness. While these findings suggest a possible role for pPTT in assessing pulmonary vascular alterations, its utility as a diagnostic tool remains under investigation, and further validation is needed before its routine clinical application can be recommended.
In 2 studies involving individuals with SLE and systemic sclerosis without established PH, pPTT was reported to be lower than in controls. A negative correlation was also observed between pPTT and both disease duration and sPAP. The authors suggested that the reduction in pPTT in early disease stages may reflect early pulmonary vascular changes.43,
Study Limitations
This study is limited by its relatively small sample size and single-center design. A single cardiologist performed TTE due to restrictions during the COVID-19 pandemic. The duration of HIV diagnosis in PWH was unknown. The PWH were more likely to be overweight, smokers, and alcohol consumers, which may have influenced echocardiographic findings. Matching between groups was not performed for potential confounding factors such as genetic background and body weight. Cardiac MRI could not be obtained due to restrictions during the COVID-19 pandemic.
Additionally, the cross-sectional design precludes the establishment of a causal relationship between HIV infection and the observed cardiac abnormalities. In the control group, diabetes mellitus and hypertension were excluded solely based on medical history, without further laboratory or imaging confirmation. Comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, and acute or chronic renal failure, which could have influenced echocardiographic findings, were excluded based on patient history and routine blood tests. However, further imaging studies were not performed due to restrictions during the COVID-19 pandemic.
Conclusion
With the advent of HAART, life expectancy among PWH has significantly increased. However, this population remains at elevated risk for CVD, highlighting the importance of early detection to reduce morbidity and mortality. In this study, certain echocardiographic parameters, such as increased IVS and PW thickness, modest elevations in E/e′ ratio, and reduced pPTT, were statistically significant in PWH compared to controls. These findings may reflect early subclinical changes in myocardial or pulmonary vascular function. However, it is important to interpret these changes with caution. Most echocardiographic parameters remained within normal limits, and the clinical relevance of borderline variations remains uncertain. While reduced pPTT was associated with CD4+ count at initial diagnosis, mitral E/A ratio post-Valsalva maneuver, and TRV after 6-MWT, the observed differences were modest and require validation in larger, longitudinal studies. Similarly, elevated E/e’ ratios in participants with CD4+ counts below 350 may suggest a trend toward DD, but fall short of indicating clinically overt heart failure. These findings suggest that echocardiographic assessment, including pPTT, E/e’, IVS, and PW thickness, could potentially serve as adjunct tools in the early evaluation of cardiovascular involvement in PWH. Furthermore, changes in diastolic indices could aid in monitoring disease progression or regression. The pPTT, in particular, may offer insight into the pulmonary vascular status and right ventricular adaptation. Nonetheless, their utility as predictive or diagnostic markers requires further investigation. Larger prospective studies using multimodal imaging and incorporating long-term clinical outcomes will be essential to determine the significance of these preliminary observations.
Footnotes
All authors have reviewed the article, contributed to the analysis and/or interpretation of the data, and provided input. All authors read and approved the final version of the article.
References
- . FACT SHEET 2024 Global HIV statistics. . ;():-. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- Granich R, Gupta S, Hersh B. Trends in AIDS deaths, new infections and ART coverage in the top 30 countries with the highest AIDS mortality burden; 1990-2013. PLoS One. 2015;10(7):e0131353-.
- Boccara F, Lang S, Meuleman C. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61(5):511-523.
- Freiberg MS, Chang CCH, Kuller LH. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614-622.
- Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol. 1987;18(12):1293-1296.
- Jonigk D, Golpon H, Bockmeyer CL. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol. 2011;179(1):167-179.
- Bigna JJR, Sime PSD, Koulla-Shiro S. HIV related pulmonary arterial hypertension: epidemiology in Africa, physiopathology, and role of antiretroviral treatment. AIDS Res Ther. 2015;12():36-.
- Isasti G, Moreno T, Pérez I, Cabrera F, Palacios R, Santos J. High prevalence of pulmonary arterial hypertension in a cohort of asymptomatic HIV‐infected patients. AIDS Res Hum Retrovir. 2013;29(2):231-234.
- Rasoulinejad M, Moradmand Badie S, Salehi MR. Echocardiographic assessment of systolic pulmonary arterial pressure in HIV-positive patients. Acta Med Iran. 2014;52(11):827-830.
- Schwarze-Zander C, Pabst S, Hammerstingl C. Pulmonary hypertension in HIV infection: a prospective echocardiographic study. HIV Med. 2015;16(9):578-582.
- Kovacs G, Bartolome S, Denton CP. Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J. 2024;64(4):2401324-.
- Quezada M, Martin-Carbonero L, Soriano V. Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS. 2012;26(11):1387-1392.
- Erdol MA, Acar B, Ertem AG. Assessment of pulmonary arterial hemodynamic and vascular changes by pulmonary pulse transit time in patients with human immunodeficiency virus infection. J Cardiovasc Echogr. 2021;31(1):6-10.
- Sims Sanyahumbi AE, Hosseinipour MC, Guffey D. HIV-infected children in Malawi have decreased performance on the 6-minute walk test with preserved cardiac mechanics regardless of antiretroviral treatment status. Pediatr Infect Dis J. 2017;36(7):659-664.
- Nagueh SF, Smiseth OA, Appleton CP. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
- . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Oursler KK, Katzel LI, Smith BA, Scott WB, Russ DW, Sorkin JD. Prediction of cardiorespiratory fitness in older men infected with the human immunodeficiency virus: clinical factors and value of the six-minute walk distance. J Am Geriatr Soc. 2009;57(11):2055-2061.
- Simonneau G, Montani D, Celermajer DS. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913-.
- Humbert M, Kovacs G, Hoeper MM. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 2022;43(38):3618-3731.
- . Calculators. . ;():-. https://csecho.ca/mdmath/
- Lang RM, Badano LP, Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
- Görgülü S, Eren M, Yildirim A. A new echocardiographic approach in assessing pulmonary vascular bed in patients with congenital heart disease: pulmonary artery stiffness. Anadolu Kardiyol Derg. 2003;3(2):92-97.
- Iloeje UH, Yuan Y, L’italien G. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV‐infected patients. HIV Med. 2005;6(1):37-44.
- Eliakim-Raz N, Prokupetz A, Gordon B, Shochat T, Grossman A. Interventricular septum and posterior wall thickness are associated with higher systolic blood pressure. J Clin Hypertens (Greenwich). 2016;18(7):703-706.
- Pieretti J, Roman MJ, Devereux RB. Systemic lupus erythematosus predicts increased left ventricular mass. Circulation. 2007;116(4):419-426.
- Wislowska M, Jaszczyk B, Kochmański M, Sypuła S, Sztechman M. Diastolic heart function in RA patients. Rheumatol Int. 2008;28(6):513-519.
- Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. 2020;100(2):603-632.
- Ferrari TCA, Albricker ACL, Gonçalves IM, Freire CMV. Schistosome- associated pulmonary arterial hypertension: a review emphasizing pathogenesis. Front Cardiovasc Med. 2021;8():724254-.
- Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992;67(3):338-349.
- Marecki JC, Cool CD, Parr JE. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174(4):437-445.
- Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333(4):1107-1115.
- Mermis J, Gu H, Xue B. Hypoxia-inducible factor-1 α/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res. 2011;12(1):103-.
- Holloway CJ, Ntusi N, Suttie J. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814-822.
- Lundgren JD, Babiker A, El-Sadr W. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197(8):1145-1155.
- Baba MM, Buba F, Talle MA, Umar H, Garbati M, Abdul H. Relationship between CD4 cell count, viral load and left ventricular function among HIV-1 infected patients asymptomatic for cardiac disease on HAART. West Afr J Med. 2021;38(6):571-577.
- Hsue PY, Hunt PW, Ho JE. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3(1):132-139.
- Mondy KE, Gottdiener J, Overton ET. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52(3):378-386.
- Butler J, Greene SJ, Shah SH. Diastolic dysfunction in patients with human immunodeficiency virus receiving antiretroviral therapy: results from the CHART study. J Card Fail. 2020;26(5):371-380.
- Menanga AP, Ngomseu CK, Jingi AM. Patterns of cardiovascular disease in a group of HIV-infected adults in Yaoundé, Cameroon. Cardiovasc Diagn Ther. 2015;5(6):420-427.
- Bouramoue C, Ekoba J. Le coeur et le Sida. Med Trop (Mars). 1996;56(3):33-39.
- Longo-Mbenza B, Tonduangu K, Kintonki Vita E, Seghers KV. Influence de l’infection par le VIH sur la fréquence elevée des cardiopathies a Kinshasa (Zaïre). Etude echocardiographique. Ann Cardiol Angeiol (Paris). 1997;46(2):81-87.
- Wibmer T, Rüdiger S, Scharnbeck D. Pulmonary pulse transit time: a novel echocardiographic indicator of hemodynamic and vascular alterations in pulmonary hypertension and pulmonary fibrosis. Echocardiography. 2015;2(6):904-911.
- Efe TH, Doğan M, Özişler C. Pulmonary arterial hemodynamic assessment by a novel index in systemic lupus erythematosus patients: pulmonary pulse transit time. Anatol J Cardiol. 2017;18(3):223-228.
- Dogan M, Efe TH, Cimen T. Pulmonary arterial hemodynamic assessment by a novel index in systemic sclerosis patients: pulmonary pulse transit time. Lung. 2018;196(2):173-178.


