2Department of Cardiology, Hacettepe University, Faculty of Medicine, Ankara, Türkiye; Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, The Netherlands
Abstract
Background: Atrial tachyarrhythmias (ATa) during the blanking period (BP) may predict late recurrences of arrhythmia. This study evaluates the outcomes of redo procedures during BP in patients with early recurrence after catheter ablation (CA) for atrial fibrillation (AF).
Methods: This retrospective study included patients undergoing redo procedures within 3 months of their initial CA due to severely symptomatic ATa episodes. Baseline data, medications, and procedural details of initial and redo CAs were analyzed from medical records.
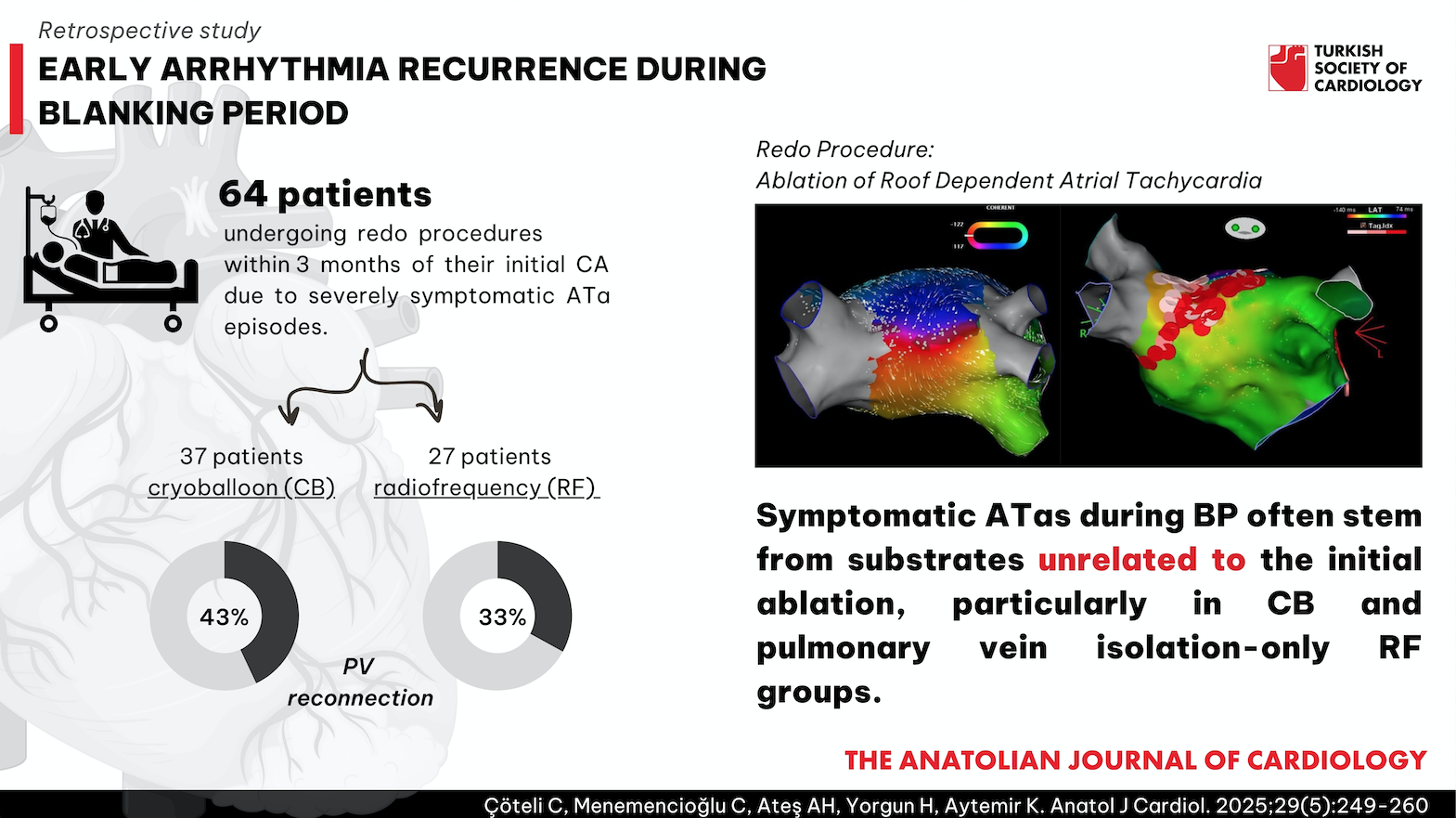
Results: Among 64 patients, 37 underwent cryoballoon (CB) and 27 underwent radiofrequency (RF) ablation. In the CB group, additional low-voltage areas beyond pulmonary veins, cavo tricuspid isthmus (CTI)-dependent flutter (27%), and left atrial reentrant tachycardia (30%) were common. Pulmonary vein reconnection was observed in 43%. In the RF group, left atrial macro/micro reentrant tachycardia (63%), CTI-dependent flutter (22%), and pulmonary vein reconnection (33%) were common causes of symptomatic ATas. After 12 months, 85.9% of patients (n = 55) were free from ATa following redo procedures.
Conclusion: Symptomatic ATas during BP often stem from substrates unrelated to the initial ablation, particularly in CB and pulmonary vein isolation-only RF groups. These findings suggest the need to reevaluate BP definitions, as select patients may benefit from early redo procedures to enhance longterm outcomes.
Graphical Abstract
Highlights
- A significant proportion of patients experiencing early recurrence after CA for AF exhibit issues such as pulmonary vein reconnection or atrial tachycardia related to left atrial scarring.
- The diversity in findings may be attributed to variations in the methodologies of the initial ablation procedures.
- The prognosis of AF is unlikely to be altered by merely waiting out the BP, given the underlying electrophysiological abnormalities.
- An algorithm tailored for patients who exhibit early recurrence during the BP is essential for enhancing long-term outcomes.
Introduction
Numerous clinical trials have demonstrated the efficacy of catheter ablation (CA) as a therapeutic approach for both paroxysmal atrial fibrillation (PAF) and persistent atrial fibrillation (PeAF).1,
Most clinical trials about CA for AF determine the recurrence as any ATa episodes occurring post the BP.4 Still, previous studies indicate that ATa episodes during BP may predict late recurrences.5,
In this study, we aimed to present findings of redo procedures performed during the BP in patients with early recurrence after CA of AF.
Methods
Study Population
In this retrospective analysis of 1482 patients who underwent CA for AF in the Hacettepe University Department of Cardiology Electrophysiology Unit between January 2017 and September 2023, 122 patients had documented ATa during the BP. Among these patients, 57 were referred for electrophysiological study (EPS) and/or redo CA due to severe symptoms [European Heart Rhythm Association (EHRA) 3-4] related to ATa episodes. Additionally, 7 patients who had their index procedure at another center were admitted to our center with ATa and referred for EPS and/or redo CA. In total, 64 patients were enrolled in the study (
In all patients, baseline characteristics, medications, and procedural details of the index and redo CAs were recorded from either patients’ files or the hospital’s electronic database.
The study was approved by Hacettepe University Non-interventional Clinical Research Ethic Committee (Date: 21/03/2023, No: 2023/05-18).
Artificial intelligence tools, including ChatGPT and Grammarly, were used to identify and correct language and grammatical errors.
Index Ablation Procedure
All patients underwent CA for AF with either cryoballoon (CB) or radiofrequency (RF) ablation. Among the 64 patients, 57 had index procedures in our hospital, and 7 were referred from another clinic for a redo procedure. In all patients who had index procedures in other centers, pulmonary vein isolation (PVI) using CB was the ablation technique.
In patients who had index procedures at our hospital, all procedures were started in sinus rhythm, and an initial EPS was performed. In the initial EPS, intravenous isoproterenol was routinely infused (0.5-4 μg/min to increase the heart rate by 25%) in patients without inducible tachycardia before referring to CA of AF.
In the CB procedure, only PVI or eLAAi (empirical left atrial appendage isolation) in addition to PVI were performed. All procedures were conducted using second- or fourth-generation 28-mm CB catheters (Arctic Front AdvanceTM and Arctic Front AdvanceTM ST, Medtronic, Minneapolis, Minn, USA). Procedural details of CB-based PVI and PVI plus eLAAi are elaborated in our previous study.7
All RF procedures were performed using 3-dimensional (3D) electroanatomical mapping systems (Carto™, Biosense Webster, and EnsiteX™, Abbott). High-density mapping catheters (Pentaray™, Biosense Webster, and The Advisor™ HD Grid, Abbott) were employed under sinus rhythm to pinpoint the substrate in the left atrium. PVI was performed using contact force irrigated RF ablation catheters (Thermocool Smarttouch™, Biosense Webster, and TactiCath™, Abbott) in all patients as the cornerstone of the CA. PVI was performed using the CLOSE protocol8 when the Carto™ system was used, and the LSI workflow9 (LSI ≥5.0) when EnsiteX™ was used. If any low voltage areas or late potentials were detected in substrate mapping (SM) or any AT induced following PVI, supplementary ablation was performed by linear or focal ablation sets. Procedural details of RF-based CA for AF are delineated in our previous study.10
Follow-Up Protocol
For patients experiencing symptomatic ATas, including any AF or AT episodes lasting longer than 30 seconds or appearing on surface ECG, during the first 3 months, 12-lead ECG recordings were performed and the EHRA symptom classification was used to evaluate symptoms. If ATas were not documented with 12 lead ECG and patients had EHRA 3-4 symptoms, 24-hour rhythm monitoring was scheduled to investigate symptomatic arrhythmias.
Following the redo procedure, patients were evaluated at 1, 3, 6, and 12 months using 12 lead ECG and 24-hour Holter monitoring to assess ATas and recurrence. Recurrences were defined as the presence of ATas on the 12 lead ECG or an episode of ATas lasting at least 30 seconds on the Holter monitor.
Patient Selection for Referral Redo Ablation Procedure
The patients with AT in surface ECG were referred to EPS and had the procedure regardless of the severity of their symptoms.
Patients with documented AF who were asymptomatic or mildly symptomatic (EHRA 2) were not referred for interventional procedures until the end of the BP unless there was tachycardia-induced cardiomyopathy. If they were in AF, cardioversion was performed, and the antiarrhythmic drug was switched to amiodarone if they were using propafenone, flecainide, or sotalol. If the patients were severely symptomatic (EHRA 3-4) and had documented ATas, they were referred to EPS and SM.
Symptomatic patients (regardless of severity) and patients with recurrent ATas who remained symptomatic despite cardioversion and medication changes were referred to EPS and SM, if necessary.
Redo Ablation Procedure
All patients were referred to the redo procedure in sinus rhythm or AT. If they were in AF on admission, they were cardioverted before the redo procedure. In patients with sinus rhythm, an initial EPS was performed to exclude supraventricular tachycardia (SVT). If atrioventricular nodal reentrant tachycardia (AVNRT) or a concealed accessory pathway was diagnosed in EPS, the aim was to ablate the slow or accessory pathway. The induction protocol with or without isoproterenol was the same as the initial procedure. If SVT was not found, but AT was induced, we continued the procedure under general anesthesia and guidance of 3D electro-anatomical mapping (CARTO 3, Biosense Webster or Ensite Precision/Ensite X, Abbott) using high-density multipolar catheters (Pentaray, Biosense Webster, and HD-Grid, Abbott). Contact force irrigated RF ablation catheters (Thermocool Smarttouch™, Biosense Webster, and TactiCath™, Abbott) were used when 3D electro-anatomical mapping systems were utilized.
In the redo procedure, the right atrium was initially mapped for activation if there was right-sided AT, according to the surface ECG and entrainment maneuvers. If the activation map indicated cavo tricuspid isthmus (CTI)-dependent AT, a CTI line was created. Following the creation of a CTI line or in patients with left-sided AT, the transseptal puncture was performed using the modified Brockenbrough technique under fluoroscopic guidance. Unfractionated heparin boluses were administered to maintain the activated clotting time (ACT) of 300-350 seconds after LA access was obtained. After the puncture, a steerable sheath was placed in the left atrium. Subsequently, the LA was mapped for scar, pulmonary vein (PV) reconnection, and also for activation. In the activation map, micro reentry is defined as ATs with a cycle length coverage greater than 85% within a 3 cm diameter circuit that activates the rest of the atrial chamber centrifugally.11 Linear ablation lines were drawn with RF energy according to the activation of AT. Radiofrequency ablation was conducted utilizing an irrigated tip contact force ablation catheter, which had an energy setting between 25W and 40W, a contact force ranging between 10 gr and 20 gr, a temperature ceiling of 42°C℃ , and a duration of between 30 seconds and 60 seconds. The earliest activation was targeted for focal ATs, while the critical isthmus was ablated in localized reentry. In the cases where activation mapping revealed macro reentry, linear lesion sets were created between anatomical obstacles and electrically isolated regions. Following the ablation sets, the bidirectional block was checked across each line. After termination of AT, PVs were isolated if PV reconnection was observed, and other sustained ATs were controlled with electrical stimulation.
If the initial rhythm was sinus, and no AT was induced, the left atrium was mapped for PV reconnection, scar, and substrate after 2 transseptal punctures. If PV reconnection was observed, PVI was performed using RF energy. If the PVs were isolated, the focus shifted to the scar and left atrial substrate. Additional atrial electrical stimulation was performed while intravenous isoproterenol was infused to induce sustained AT. If there was no sustained AT, substrate modification was performed.
Left atrial substrate evaluation was made with late potential mapping. Functional SM using late potential mapping was described in our previous study 10. In summary, isochronal late activation mapping (ILAM) was established during either sinus or paced rhythm by annotating the offset of the latest atrial deflections. The time window for examining atrial activation was determined to be between the onset of the earliest and offset of the latest local EGMs recorded by a high-density mapping catheter in the left atrium. Subsequently, the entire activation process was divided into 8 evenly distributed time intervals, known as isochrones. Regions displaying isochronal crowding, defined as deceleration zone (DZ), were identified as areas where more than 3 colors representing isochronal lines were observed within a 1 cm radius. Additionally, fragmented electrical signals were evaluated in the left atrium and marked in distinct colors. "Fragmented EGM" was defined as having at least 4 distinct fluctuations in the atrial bipolar electrogram from the isoelectric baseline.12 Simultaneously, SM was carried out with the following voltage criteria: scar, ≤0.2 mV; border zone, 0.2-0.5 mV; normal, >0.5 mV.13 If extensive scarring (low voltage area presence of more than 1 left atrial wall including anterior, posterior, roof, and septum) was present, substrate ablation was done using low voltage zones with DZs and fragmented EGMs as box or linear lesions.
In the case of an electrically healthy atrium with isolated PVs, an intravenous bolus of isoproterenol (3-5 μg) was used to trigger AF. If a non-PV trigger (ectopic beats initiating ATa) was identified, it was trapped using RF. Focal AT within the SVC is defined as active SVC; in these patients, the SVC was isolated.
Statistical Analyses
The statistical analysis was conducted using Stata (version 18.5; StataCorp LLC, College Station, Tex, USA). The normality of variable distributions was assessed using the Shapiro-Wilk test. Quantitative variables with a Gaussian distribution were reported as mean and SD, while variables with a non-Gaussian distribution were reported as median (minimum and maximum). Categorical data were presented as numbers and percentages. Follow-up data were analyzed and visualized using Kaplan-Meier survival curves.
Results
Basal Characteristics
A total of 64 patients were enrolled in the study, 35 (54.7%) were female. The mean age of the study population was 60 ± 13. 43 patients (67.2%) had a diagnosis of PAF before undergoing the index procedure. The mean LA diameter was 40 ± 5 mm, and the median AF duration was 8 months (1-120 months). The median CHA2DS2-VASc score for the group was calculated to be 2 (0-6). Detailed demographic characteristics are shown in
Index Procedure
During the index procedure, 37 (57.8%) patients underwent CB for PVI, whereas 27 (42.2%) patients did not. The index ablation was performed at our center in 57 (89.1%) patients. In addition to the PVI, 5 patients underwent LAA isolation in the CB ablation group.
Among the 27 patients subjected to CA using RF, additional ablation lines or substrate modification were executed as an adjunct to PVI in 13. LA macro reentrant tachyarrhythmias were induced with atrial stimulation following PVI in 7 of these patients. For these patients, linear ablations were created with the anterior line in 3 for perimitral AT and the roof line in 4 for roof-dependent AT, in addition to PVI. Additionally, LA SM in 7 patients revealed late potentials and continuous fragmented electrograms; the posterior box was drawn in 2 individuals, and the LA substrate was modified in 5. Cavo tricuspid isthmus-dependent typical atrial flutter was identified in 1 patient, and a CTI line was created. Left atrial appendage was found to be active in another patient, and LAA was isolated.
The study population was divided into 4 distinct groups: only PVI with CB (Group 1), PVI and empirical LAA isolation with CB (Group 2), only PVI with RF (Group 3), and PVI and additional ablation lines/isolation with RF (Group 4).
Details of the index procedures are shown in
Diagnosis of Atrial Tachyarrhythmias during the Blanking Period
Following the initial procedure, all patients were administered anticoagulants. Antiarrhythmic drugs were prescribed in 44 (68.7%) patients (
Redo Procedure
Follow-Up
During the 12-month follow-up period, 9 patients (14.1%) experienced episodes of ATa during the 12-month follow-up period. No mortality or major adverse cardiac events were observed. The index procedures included PVI with CB in 3 patients, PVI and LAAi with CB in 2, only PVI with RF in 1, and PVI with additional lesions in 3. When comparing follow-up results in the 2 different groups according to the index procedure, no statistically significant difference in survival was observed between the groups, as per the log-rank test results [
Discussion
Our study’s principal finding is the notable proportion of patients who encounter symptomatic ATa episodes during the BP and exhibit evidence of PV reconnection or underlying substrates that may contribute to the recurrence of ATas. This observation raises a pivotal question about the BP’s genuine validity. If the BP holds significance, it beckons another query: which patients should we monitor until the end of the BP before confirming a recurrence?
Mechanism of Early Recurrence and Duration of Blanking Period
The definition of BP remains a topic of debate among both patients and physicians.14,
The primary rationale behind the BP is ablation-related inflammation.17 As the scar matures, the iatrogenic lesions initially have proarrhythmic effects, which are expected to subside once the scarring process stabilizes and a homogeneous scar forms. Uetake et al18 emphasize the role of inflammation in early recurrence, noting that it can persist for up to 2 months. However, due to the variability in individual and procedural characteristics, it is difficult to estimate the exact duration of the BP, which likely explains the differing BP durations reported in various studies.
Antiarrhythmic drugs (AADs) may mitigate episodes of inflammation-related ATa during BP.16 However, some ATa episodes may persist and continue to cause symptoms. Recent studies indicate that ATa episodes during BP, although not classified as recurrences, are associated with long-term recurrences.4,
Atrial Tachycardia-Related Early Recurrence
In our study, patients were categorized into 4 groups based on their index procedure to assess the impact of the index procedure on the outcomes of the second procedure. In the group that underwent the index procedure with CB (n = 37), AT was present or induced during the second procedure in a significant portion of this subgroup. Cavo tricuspid isthmus-dependent AFl was identified as the cause of AT in 10 patients, and re-entrant left AT in 11. These instances of CTI-dependent AFl and scar-related left AT are presumed to be unrelated to the initial ablation. However, previous studies have reported conflicting results on the efficacy of empirical CTI ablation in improving outcomes for AF recurrences.22,
On the other hand, scar-related left AT might have a role in initiating AF. Previous studies showed that left atrial scar is an independent risk factor for recurrences.26 Yet, previous studies have not demonstrated a definitive benefit of scar-based CA, and consensus on its use for AF management remains elusive.27,
For patients with a history of RF-based CA, admitted with left AT, or those in whom left AT was induced during the EPS, formulating recommendations becomes complex due to 2 potential etiologies for tachycardia. First, it might stem from the aftereffects of a prior ablation procedure. In our group 3, the patients who had PVI using RF (n = 14), 8 patients encountered AT during the second procedure. This included 6 with macro-reentrant AT, 1 micro-reentrant, and 1 focal AT. What is intriguing here is that no left atrial substrate was identified in the preliminary procedure; only PVI was performed. This brings to light the notion that some ATs might result from the functional slow conduction zones, iatrogenically created in the initial procedure. Waiting for the scar maturation might be an option for some patients before electing a redo procedure, particularly with localized reentry and electrical substrate on the initial ablation lines. However, the implications of delaying intervention in patients with macro reentry remain uncertain, and we think early termination of macro reentry via RF ablation in these patients would improve long-term results. Secondly, AT episodes could be related to the previously present left atrial substrate. In group 4, the patients with PVI and additional ablation lines using RF (n = 13), 10 patients faced left AT in the second procedure. Masuda et al29 reported that linear ablation lines, in addition to PVI, significantly improve follow-up. However, they also reported that linear lesions can increase iatrogenic ATs. Thus, the outcomes for each patient must be interpreted in the context of their primary procedure. This suggests that waiting till the end of BP would not alter their prognosis, maintaining a heightened risk of recurrence.
Pulmonary Vein Reconnection-Related Early Recurrence
Managing PV reconnection within the initial 3-month post-ablation period remains a debated issue. The etiology of PV reconnection is not entirely elucidated; however, multiple hypotheses have been posited. A primary theory suggests that PV electrical reconnection may stem from an incomplete encirclement of the PV ostia, creating a gap that permits the re-establishment of electrical activity.30 Ranjan et al31 demonstrated how discontinuities in the ablation line can facilitate the restoration of electrical isolation by employing magnetic resonance imaging. Similarly, Kowalski et al32 found that nontransmural ablation lesions are associated with PV reconnection. A second hypothesis centers on the concept of dormant conduction, which is postulated to be a predominant factor in early PV reconnection.33 The use of adenosine to reveal dormant conduction has been documented; however, the clinical significance of employing the adenosine test for this purpose remains ambiguous.34,
Supraventricular Tachycardia-Related Recurrence
In our study population, AVNRT was induced during the initial EPS of the second procedure in 5 (7.8%) patients. Previous studies have reported that AVNRT and atrioventricular reentrant tachycardia (AVRT) might degenerate into AF.36,
To the best of our knowledge, our study is the first clinical study to comprehensively evaluate additional substrates beyond PVs in patients with incessant ATa during BP. The findings of this trial may challenge the conventional belief about BP and raise new questions. Although early recurrences are not typically considered recurrences in clinical trials, waiting until the end of BP may decrease the success rate of CA.
Clinical Approach for Patients with Early Recurrence Following Catheter Ablation for Atrial Fibrillation
Upon reviewing these findings, we propose a new algorithm (
Upon admission, the initial step involves thoroughly evaluating the patient's symptoms and analyzing their 12-lead surface ECG.
In patients with EHRA 2 symptoms, an ECG might guide the next steps. If the rhythm is sinus but there are PAF episodes within 24 hours of rhythm monitoring, waiting until the end of the BP might be an option. Similarly, cardioversion and waiting for the BP might be options for patients with AF. However, in patients with AT rhythm, they can be referred to a redo procedure.
In patients with EHRA 3-4 symptoms or tachycardia-induced cardiomyopathy (regardless of symptoms), it is recommended to proceed with an EPS and, if necessary, SM for those presenting with a sinus rhythm. Activation mapping should be the course of action in cases where AT is observed. When AF is detected, the protocol involves cardioverting the patient before referring them for an EPS and potentially further SM.
If the EPS identifies AVNRT or the presence of an accessory pathway, the appropriate response is to carry out an ablation of the implicated slow pathway or the accessory pathway, respectively. When AT is provoked during the EPS, activation mapping specifically for AT should be executed.
In scenarios where the EPS does not yield any particular findings, the focus shifts to controlling PVI and conducting a map for any underlying substrate. Furthermore, if the admission rhythm is AT or if AT is induced during EPS and ablation is successfully completed, it is necessary to verify PVI and perform SM.
If PV reconnection is observed, the pulmonary veins must be isolated. Lastly, if SM uncovers a left atrial scar without additional anomalies, it might be beneficial to consider drawing additional ablation lines.
This proposed algorithm provides a clear and concise approach to managing patients who have experienced severely symptomatic ATa episodes during the BP. It emphasizes the importance of performing an EPS and SM to determine the underlying rhythm and identify potential ablation targets. It also highlights the need for thorough follow-up and assessment of PVI and SM after successful ablation. On the other hand, this proposed algorithm should be evaluated in controlled trials with larger study groups before being routinely used for patients with early recurrence following CA for AF.
Our findings are consistent with those reported by Ganesan et al39 in their meta-analysis, where a long-term success rate of 79% was observed with multiple procedures. Similarly, in our study, during the 12-month follow-up period, 85.9% of patients remained free from ATa. This favorable outcome highlights the effectiveness of our approach in maintaining arrhythmia control, even in patients with early recurrences.
Patient Selection for Referral Redo Procedure
This trial suggests that an early ablation strategy might benefit patients with early recurrence within the first 3 months following CA for AF. However, the STOP AF trial40 indicated that a significant portion of patients with early recurrence would not experience late recurrence, making ablation-related risks potentially unnecessary for these patients. Consequently, patient selection for early ablation procedures should be done carefully and based on clear criteria. The novel consensus document19 on CA for AF recommends an early re-ablation strategy only for severely symptomatic patients. Future randomized clinical trials are needed to establish more definitive criteria for early ablation strategies.
Study Limitations
Several limitations to this trial should be noted. First, it is important to note that this is a retrospective study with a relatively small sample size. Therefore, specific recommendations for this patient group may not be possible based solely on these findings. Future large-scale clinical trials are needed to make more definitive recommendations. Second, the study population was heterogeneous, consisting of patients with PAF and PeAF who underwent various types of ablation procedures, including CB and RF ablation, as well as PVI and PVI plus additional ablative strategies. Therefore, it's important to emphasize that these patient characteristics may lead to different endpoints at the end of the BP. Another clinically significant limitation is the uncertainty regarding the clinical impact of ablation-induced inflammation. It is unclear whether the tachycardia we induced is the same as the clinical tachycardia. There is no definitive evidence to determine if the induced tachycardia is a result of inflammation related to the index procedure's ablation. Lastly, there is no clear recommendation for the usage of AADs during the BP for the prevention of early recurrence. AADs can reduce inflammation-related early recurrence within the BP. However, the AAD usage following the index procedure was relatively low in our study population.
Conclusion
This study revealed that many patients who experienced ATa during the BP have PV reconnection and left atrial scar-related AT. Furthermore, most of these ATs are unrelated to the index procedure or the results of the initial ablation sets. The algorithm we used for this specific patient population could help physicians reduce recurrences and symptomatic episodes following CA. Further randomized controlled studies with longer follow-up periods will provide more insight into this complex issue.
Footnotes
References
- Poole JE, Bahnson TD, Monahan KH. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA Trial. J Am Coll Cardiol. 2020;75(25):3105-3118.
- Wazni OM, Dandamudi G, Sood N. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324.
- Calkins H, Hindricks G, Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20(1):e1-e160.
- Amuthan R, Curtis AB. What clinical trials of ablation for atrial fibrillation tell us – and what they do not. Heart Rhythm. 2021;2(2):174-186.
- Arya A, Hindricks G, Sommer P. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. EP Europace. 2009;12(2):173-180.
- Themistoclakis S, Schweikert RA, Saliba WI. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008;5(5):679-685.
- Yorgun H, Canpolat U, Kocyigit D, Çöteli C, Evranos B, Aytemir K. Left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation: one-year clinical outcome after cryoballoon-based ablation. Europace. 2017;19(5):758-768.
- Taghji P, El Haddad M, Phlips T. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: A pilot Study. JACC Clin Electrophysiol. 2018;4(1):99-108.
- Venkatesh Prasad K, Bonso A, Woods CE. Lesion Index-guided workflow for the treatment of paroxysmal atrial fibrillation is safe and effective - Final results from the LSI workflow Study. Heart Rhythm. 2022;3(5):526-535.
- Yorgun H, Çöteli C, Kılıç GS. Functional substrate mapping characteristics during sinus rhythm predicts critical isthmus of reentrant atrial tachycardia. J Cardiovasc Electrophysiol. 2023;34(7):1539-1548.
- Mantovan R, Corò L, Allocca G, Sitta N, Rivetti L, Marinigh R. How small could a detectable reentrant circuit be in a localized microreentrant tachycardia?. HeartRhythm Case Rep. 2020;6(4):222-225.
- Jadidi AS, Duncan E, Miyazaki S. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012;5(1):32-42.
- Herczeg S, Walsh K, Keaney JJ. Quantitative assessment of left atrial scar using high-density voltage mapping and a novel automated voltage analysis tool. J Interv Card Electrophysiol. 2020;59(1):5-12.
- Bordignon S, Barra S, Providencia R. The blanking period after atrial fibrillation ablation: an European Heart Rhythm Association survey on contemporary definition and management. Europace. 2022;24(10):1684-1690.
- Willems S, Khairy P, Andrade JG. Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation: Insights From the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ Arrhythm Electrophysiol. 2016;9(8):-.
- Joglar JA, Chung MK, Armbruster AL. ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation. J Am Coll Cardiol. 2023;0(0):-.
- Alipour P, Azizi Z, Pirbaglou M. Defining blanking period post-pulmonary vein antrum isolation. JACC Clin Electrophysiol. 2017;3(6):568-576.
- Uetake S, Miyauchi Y, Mitsuishi T, Maruyama M, Seino Y, Shimizu W. Re-definition of blanking period in radiofrequency catheter ablation of atrial fibrillation in the contact force era. J Cardiovasc Electrophysiol. 2020;31(9):2363-2370.
- Tzeis S, Gerstenfeld EP, Kalman J. . EP Europace. 2024;2024():-.
- Liang JJ, Elafros MA, Chik WW. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm. 2015;12(12):2461-2468.
- Evranos B, Aytemir K, Oto A. Predictors of atrial fibrillation recurrence after atrial fibrillation ablation with cryoballoon. Cardiol J. 2013;20(3):294-303.
- Romero J, Patel K, Briceno D. Cavotricuspid isthmus line in patients undergoing catheter ablation of atrial fibrillation with or without history of typical atrial flutter: A meta-analysis. J Cardiovasc Electrophysiol. 2020;31(8):1987-1995.
- Mesquita J, Ferreira AM, Cavaco D. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol. 2018;259():82-87.
- Gupta D, Ding WY, Calvert P. Cryoballoon pulmonary vein isolation as first-line treatment for typical atrial flutter. Heart. 2023;109(5):364-371.
- Romero J, Di Biase L. Empirical cavotricuspid isthmus line for atrial fibrillation ablation is futile "Repetita Iuvant". Int J Cardiol. 2018;259():107-108.
- Verma A, Wazni OM, Marrouche NF. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45(2):285-292.
- Masuda M, Asai M, Iida O. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO Trial. J Am Heart Assoc. 2020;9(13):-.
- Marrouche NF, Wazni O, McGann C. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical Trial. JAMA. 2022;327(23):2296-2305.
- Masuda M, Inoue K, Tanaka N. Long‐term impact of additional ablation after pulmonary vein isolation: results from EARNEST‐PVI Trial. J Am Heart Assoc. 2023;12(17):e029651-.
- McGarry TJ, Narayan SM. The anatomical basis of pulmonary vein reconnection after ablation for atrial fibrillation: wounds that never felt a scar?. J Am Coll Cardiol. 2012;59(10):939-941.
- Ranjan R, Kato R, Zviman MM. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol. 2011;4(3):279-286.
- Kowalski M, Grimes MM, Perez FJ. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol. 2012;59(10):930-938.
- Cheema A, Dong J, Dalal D. Incidence and time course of early recovery of pulmonary vein conduction after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18(4):387-391.
- Iqbal M, Jena A, Park HS. Value of adenosine test to reveal dormant conduction or adenosine-induced atrial fibrillation after pulmonary vein isolation. J Arrhythm. 2017;33(6):602-607.
- Chen C, Li D, Ho J. Clinical implications of unmasking dormant conduction after circumferential pulmonary vein isolation in atrial fibrillation using adenosine: A systematic review and meta-analysis. Systematic review. Front Physiol. 2019;17():-.
- Sauer WH, Alonso C, Zado E. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: Response to ablation that incorporates slow-pathway modification. Circulation. 2006;114(3):191-195.
- Della Bella P, Brugada P, Talajic M. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356.
- Wutzler A, von Ulmenstein S, Attanasio P. Where there's smoke, there's fire? Significance of atrial fibrillation in young patients. Clin Cardiol. 2016;39(4):229-233.
- Ganesan AN, Shipp NJ, Brooks AG. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):-.
- Andrade JG, Khairy P, Macle L. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal atrial fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014;7(1):69-75.


