2Department of Cardiology, İstanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital, İstanbul, Türkiye
3Department of Cardiovascular Surgery, İstanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital, İstanbul, Türkiye
Abstract
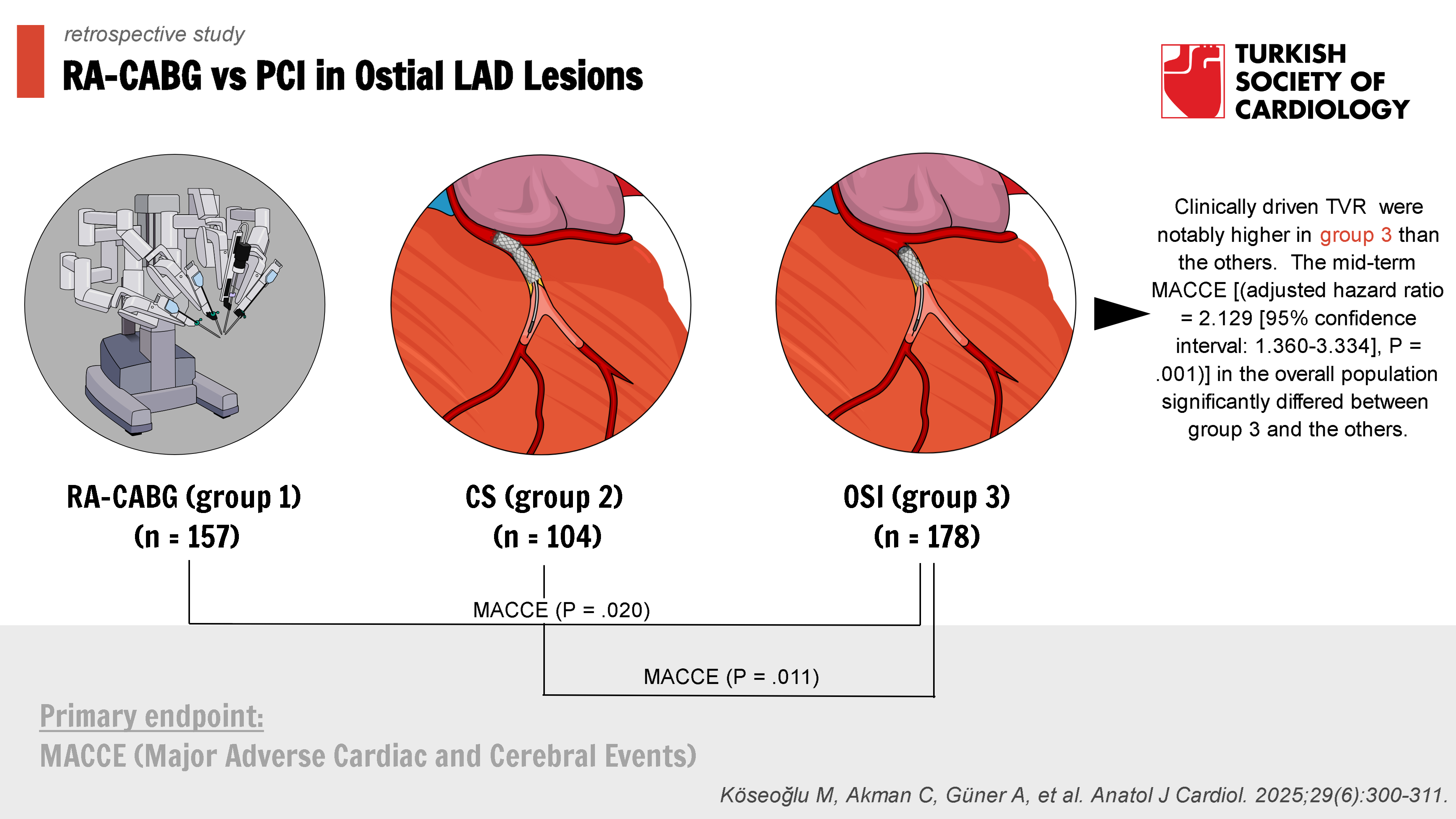
Background: The comparison of outcomes of robotic-assisted coronary artery bypass grafting (RA-CABG) vs. stenting techniques (ostial or crossover stenting) for ostial left anterior descending (LAD) artery lesions is still lacking. This retrospective study sought to determine the midterm outcomes of RA-CABG, crossover stenting (CS), and ostial stent implantation (OSI) in patients with ostial LAD disease.
Methods: All cases were divided into 3 groups as follows: RA-CABG (group 1) (n = 157), CS (group 2) (n = 104), and OSI (group 3) (n = 178). The primary endpoint was defined as the major adverse cardiac and cerebral events (MACCE), which included cardiac death, target vessel myocardial infarction, target vessel revascularization (TVR), stroke, and stent thrombosis or symptomatic graft occlusion during follow-up. This is the first investigation comparing midterm outcomes of RA-CABG, CS, and OSI as revascularization options for ostial LAD lesions.
Results: A total of 439 consecutive individuals [male: 341 (77.6%), mean age: 59.58 ± 9.35 years] with ostial LAD disease were included in this study. The rates of MACCE (P = .020 for groups 3 vs. 1; P = .011 for groups 3 vs. 2) and clinically driven TVR (15.7 vs. 4.5%, P = .001 for groups 3 vs. 1; 15.7 vs. 5.8%, P = .014 for groups 3 vs. 2) were notably higher in group 3 than the others. The mid-term MACCE [(adjusted hazard ratio = 2.129 [95% confidence interval: 1.360-3.334], P = .001)] in the overall population significantly differed between group 3 and the others.
Conclusion: The findings of the study suggest that OSI for ostial LAD lesions was associated with higher midterm MACCE and TVR rates than revascularization with RA-CABG or CS.
Graphical Abstract
CONTENT
Highlights
- The present study demonstrated that revascularization with either RA-CABG or CS strategies for ostial LAD disease was associated with lower MACCE and TVR rates compared with OSI, whereas other endpoints including mortality were comparable between the 3 groups.
- Crossover stenting technique appears to be a more feasible strategy than RA-CABG in terms of length of hospital stay and several intraprocedural non-fatal major complications (i.e., pneumothorax).
- In contemporary clinical practice, for Medina 0.1.0 left main bifurcation lesions, CS may be considered a viable alternative to OSI and RA-CABG approaches in patients with SYNTAX scores < 33, and we advocate the assessment of multidisciplinary collaboration in the decision-making.
- Larger prospective studies are needed to guide the management of patients with ostial LAD lesions.
Introduction
Atherosclerotic plaque distribution in the coronary vascular bed can take on a very intricate pattern in ostial left anterior descending (LAD) coronary artery disease.1 Despite invasive coronary angiography identifying these diseases as simple Medina 0.1.0, it is typically difficult to predict the inclusion of distal left main coronary artery (LMCA) disease.2-
Conventional CABG is an efficient revascularization method for complex coronary artery disease; however, it is an extremely invasive procedure that necessitates sternum dissection to access the heart. Consequently, it causes extensive scarring, a lengthy recovery period after surgery, and morbidity.8,
Methods
Study Design and Population
This retrospective observational study included 439 patients who underwent PCI or RA-CABG for ostial LAD disease between January 2011 and March 2024 in our institute. Demographic information, clinical and angiographic aspects of the patients, and cardinal symptoms were collected retrospectively. Individuals diagnosed with ST-segment elevation myocardial infarction, significant LMCA disease (30-50%), incomplete revascularization, a bare metal stent, end-stage kidney disease requiring hemodialysis, cardiogenic shock status, premature discontinuation of dual antiplatelet therapy, lost to follow-up, <1-year life expectancy, and absence of medical records were excluded from the study. We allocated the patient population into 3 groups based on the proposed revascularization strategy, including RA-CABG (group 1) as surgical intervention, and PCI therapies including either CS (group 2) or OSI (group 3). The final analysis comprised 439 patients, of which 157 were assigned to group 1, 104 to group 2, and 178 to group 3.
Interventional Procedures
Participating primary operators were required to perform 300 PCI per year for 3 years. The stents that were implanted with OSI or CS technique in the LMCA to LAD position were second- or third-generation DES, such as Firehawk (MicroPort Scientific Inc., Shanghai, China), Promus (Boston Scientific Inc., Galway, Ireland), Xience (Abbott Inc., Abbott Park, Illinois, USA), and Biomime (Meril Inc., Vapi, Gujarat, India), according to current recommendations from the EBC and at the operators’ discretion.11,
Under general anesthesia, the second, fourth, and sixth intercostal gaps were used to establish 3 ports in the anterior axillary line, and the da Vinci Xi robotic system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was introduced to begin the RA-CABG procedure. A camera equipped with surgical tools was introduced into the left pleural space along with 2 lateral arms. Under constant carbon dioxide insufflation, the left internal thoracic artery (LITA) was correctly collected. The side branches of the LITA graft were harvested using bipolar cautery forceps and a low-energy monopolar electrocautery spatula. In a semi-skeletonized fashion, the LITA and its associated veins were extracted. Using a suction stabilizer (Octopus Nuvo Tissue Stabilizer, Medtronic, Minneapolis, USA), the pericardial fat was removed under endoscopic control. The LAD was then located and an optimal anastomosis site was determined. The beating heart underwent the off-pump anastomosis to the LAD. In circumstances where many veins were involved, the appropriate harvesting vessels were the great saphenous vein or the radial artery. Anastomosing the target vessel to the venous graft or radial artery followed the same procedure. Upon arrival at the intensive care unit, the patient was intubated and will be extubated within the next several hours. Following interventional procedures, the duration of dual antiplatelet therapy, including a P2Y12 receptor inhibitor, was determined depending on the clinical status of the patient, by current major cardiovascular guidelines.9
Clinical Assessment
Intraprocedural or in-hospital major adverse events (including death) were assessed at the time of the event or before hospital discharge. At 1, 6, and 12 months following the index procedure (RA-CABG, CS, or OSI), in addition to yearly clinic visits thereafter, all patients were routinely evaluated. Exercise stress testing, myocardial perfusion scintigraphy, or echocardiography was routinely done 6 to 12 months following the intervention to measure induced myocardial ischemia, and all patients were expected to comply with routine clinic visits. Invasive coronary angiography (coronary computed tomography angiography if needed) was performed on all patients who had clinical symptoms or instrumental evidence of myocardial ischemia during the follow-up period. Clinic visits, phone calls (for event confirmation from medical records), and referrals from other doctors all contributed to in-depth evaluations of patients’ health. We also checked the National Death Database to make sure the patients’ lives or deaths were accurately recorded.
Definitions and Study Outcomes
All-cause/cardiac death, clinically driven target vessel revascularization (TVR), spontaneous target vessel myocardial infarction (TVMI), stent thrombosis, symptomatic graft occlusion, stroke, contrast-induced acute kidney injury, and major bleeding were defined according to the EXCEL trial.17 The primary outcome was measured as major adverse cardiovascular and cerebral events (MACCE), which were cardiac death, spontaneous TVMI, TVR, stroke, and stent thrombosis or symptomatic graft occlusion under mid-term follow-up.
Statistical Analysis
Descriptive statistics were reported using mean ± standard deviation for continuous variables, and frequency with percentages for categorical data. The Shapiro–Wilk and Kolmogorov–Smirnov tests were used to determine the normality of the distribution of continuous variables. All continuous variables did not show normal distribution, and therefore non-parametric tests were used. For 2-group comparisons, continuous variables were compared across groups using the Mann–Whitney
To account for treatment selection bias, the Cox proportional hazard regression model for mid-term MACCE was adjusted using the inverse probability weighted (IPW) approach to account for treatment selection bias. A multivariable logistic regression model was built to estimate the probability (propensity) of a patient being included in the OSI group versus others, given a set of measured covariates that would be predictive of the binary outcome (age, hypertension, EuroSCORE II, active side branch protection, and side branch narrowing > 50%). The standard errors of the hazard ratios from the IPW-adjusted Cox proportional hazard models are based on the robust sandwich variance estimator. Adjustment variables for both standard and IPW-adjusted Cox proportional hazard regression models were treatment (OSI), current smoker, EuroSCORE-II score, SYNTAX score, and in-hospital non-fatal major adverse events. The association between treatment and outcomes was quantified by univariate Cox regression and multiple Cox regression analyses hazard ratio (HR) and 95% confidence interval obtained by IPW-adjusted regression models. The Kaplan–Meier survival curve was used to demonstrate the cumulative incidence of MACCE, and log-rank tests were employed for group comparisons. The significance level was accepted as
Results
A total of 439 consecutive patients [male: 341 (77.6%), mean age: 59.58 ± 9.35] with ostial LAD disease were enrolled in this study. The baseline demographic, clinical, medications, and lesion characteristics of the groups are depicted in
Procedural details of the MICS-CABG and PCI arms are summarized in
In-hospital complications and mid-term clinical outcomes are presented in
In the IPW-Cox proportional hazard analysis, the mid-term MACCE [(unadjusted HR: 1.706, [95% CI: 1.115-2.611],
Kaplan–Meier analysis showed that mid-term MACCE-free survival was found to be significantly decreased in patients with the treated OSI (Log Rank
Discussion
This is the first investigation comparing mid-term outcomes of RA-CABG, CS, and OSI revascularization options for ostial LAD disease, thereby most effectively reflecting real-world practice. The 4 major findings of the present study are: (1) the primary endpoint (MACCE) developed in 86 cases (19.6%) during the mean follow-up time of 39.41 ± 21.1 months; (2) RA-CABG and CS revascularization strategies had lower ischemia-driven combined outcome (MACCE) and TVR rates compared with the OSI under mid-term follow-up; (3) RA-CABG had longer hospital lengths of stays and a higher rate of procedural complications (i.e., pneumothorax) than PCI techniques; and (4) being in group 3 (OSI) was found to be one of the independent predictors of the mid-term MACCE.
The management algorithm of ostial LAD disease remains a controversial issue, especially in the rapidly developing stent technology and RA-CABG. The decision to use one revascularization technique over the other may depend on a better understanding of the lesion complexity of the atherosclerotic plaques.2,
We observed that MACCE and clinically driven TVR occurred more frequently in the OSI group compared to the RA-CABG and CS groups. However, other endpoints were comparable between the groups. Several investigators previously indicated that minimally invasive surgical approaches and PCI had no significant difference in the combined ischemia-driven outcomes in patients with isolated LAD lesions (ostial or proximal localization) under mid- to long-term follow-up.4,
The major drawback of a minimally invasive approach (RA-CABG) is its limited indication for revascularization. Specifically, young patients with proximal LAD disease, chronic total occlusion of the ostial LAD, advanced age or comorbidities (e.g., end-stage chronic kidney disease) with ostial LAD disease, and (4) special situations in which patients decline conventional CABG with sternotomy despite the heart team's favorable opinion regarding the benefits of conventional CABG over PCI. These concerns prevent the clinical outcomes of this surgical approach (RA-CABG) from being generalized to all coronary artery lesions.10,
SYNTAX score and EuroSCORE-II are well-established scoring systems for predicting preoperative adverse events.33-
Study Limitations
Several limitations should be addressed in the present study. First of all, the non-RCT and retrospective design could be considered a major limitation that might have introduced selection bias. Therefore, we used an IPW-Cox method to minimize such a possibility. Second, this was a single-center study; therefore, these results should be cautiously generalized to other centers. Third, the sample size of the study was relatively small. However, it is still remarkable for its size within each group. Fourth, the relatively low rate of imaging in the current complex PCI climate is also a significant limitation. Fifth, preoperative coronary computed tomography angiography for LAD assessment was not routinely performed. Lastly, the determination of the severity of lesions through quantitative coronary angiographic study and regular physiological examinations is lacking.
Conclusıon
In conclusion, for patients with ostial LAD lesions, the CS and RA-CABG strategies may result in fewer mid-term combined ischemia-driven outcomes (MACCE) and clinically driven TVR rates. However, there is no appreciable difference in mid-term survival for all revascularization options. Although RA-CABG is less invasive and has better clinical outcomes than other surgical revascularization options, PCI with CS has comparable ischemia-driven outcomes and more favorable outcomes in terms of length of hospital stay and procedural complications compared to RA-CABG. Therefore, we advocate revascularization with the CS strategy in Medina 0.1.0 left main bifurcation patients with low and intermediate SYNTAX scores and emphasize the evaluation of the multidisciplinary cardiac team in the decision-making process. Our findings provide more accurate and generalizable estimates with new insights regarding patients with ostial LAD lesions. Nevertheless, large-scale prospective RCTs with the routine use of intravascular imaging tools are warranted to address the best revascularization option in complex groups of patients.
Footnotes
This study was approved by the Ethics Committee of İstanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital (Approval No: 2024.06-61, Date: 19.11.2024).
References
- Burzotta F, Lassen JF, Lefèvre T. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16(16):1307-1317. https://doi.org/10.4244/EIJ-D-20-00169
- Kovacevic M, Burzotta F, Srdanovic I, Petrovic M, Trani C. Percutaneous coronary intervention to treat unprotected left main: common (un-answered) challenges. Kardiol Pol. 2022;80(4):417-428. https://doi.org/10.33963/KP.a2022.0078
- Li S, Zhang H, Xiao C, Wang R, Wu Y. Robotically assisted coronary artery bypass graft surgery versus drug-eluting stents for patients with stable isolated proximal left anterior descending disease. J Card Surg. 2021;36(6):1864-1871. https://doi.org/10.1111/jocs.15433
- Patel NC, Hemli JM, Seetharam K. Minimally invasive coronary bypass versus percutaneous coronary intervention for isolated complex stenosis of the left anterior descending coronary artery. J Thorac Cardiovasc Surg. 2022;163(5):1839-1846.e1. https://doi.org/10.1016/j.jtcvs.2020.04.171
- Güner A, Akman C, Çiloğlu K. Long-term evaluation of revascularization strategies for medina 0.1.0 left main bifurcation lesions: the LM-CROSSOVER registry. Angiology. 2023;():33197231213194-. https://doi.org/10.1177/00033197231213194
- Lee HM, Nam CW, Cho YK. Long-term outcomes of simple crossover stenting from the left main to the left anterior descending coronary artery. Korean J Intern Med. 2014;29(5):597-602. https://doi.org/10.3904/kjim.2014.29.5.597
- Soylu K, Yıldırım U, Nasifov M. Evaluation of long-term outcomes of crossover or focal ostial stenting of left anterior descending artery ostial stenosis. Anatol J Cardiol. 2022;26(11):827-831. https://doi.org/10.5152/AnatolJCardiol.2022.1122
- Davidson LJ, Cleveland JC, Welt FG. A practical approach to left main coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80(22):2119-2134. https://doi.org/10.1016/j.jacc.2022.09.034
- Neumann FJ, Sousa-Uva M, Ahlsson A. 2018 . Eur Heart J. 2019;40(2):87-165. https://doi.org/10.1093/eurheartj/ehy394
- Gaudino M, Bakaeen F, Davierwala P. New strategies for surgical myocardial revascularization. Circulation. 2018;138(19):2160-2168. https://doi.org/10.1161/CIRCULATIONAHA.118.035956
- Burzotta F, Lassen JF, Banning AP. Percutaneous coronary intervention in left main coronary artery disease: the 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14(1):112-120. https://doi.org/10.4244/EIJ-D-18-00357
- Albiero R, Burzotta F, Lassen JF. Treatment of coronary bifurcation lesions, part I: implanting the first stent in the provisional pathway. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention. 2022;18(5):e362-e376. https://doi.org/10.4244/EIJ-D-22-00165
- Finet G, Derimay F, Motreff P. Comparative analysis of sequential proximal optimizing technique versus kissing balloon inflation technique in provisional bifurcation stenting: fractal coronary bifurcation bench test. JACC Cardiovasc Interv. 2015;8(10):1308-1317. https://doi.org/10.1016/j.jcin.2015.05.016
- Burzotta F, Louvard Y, Lassen JF. Percutaneous coronary intervention for bifurcation coronary lesions using optimised angiographic guidance: the 18th consensus document from the European Bifurcation Club. EuroIntervention. 2024;20(15):e915-e926. https://doi.org/10.4244/EIJ-D-24-00160
- Lotfi A, Jeremias A, Fearon WF. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the society of cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2014;83(4):509-518. https://doi.org/10.1002/ccd.25222
- Chieffo A, Latib A, Caussin C. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65-72. https://doi.org/10.1016/j.ahj.2012.09.017
- Kappetein AP, Serruys PW, Sabik JF. Design and rationale for a randomised comparison of everolimus-eluting stents and coronary artery bypass graft surgery in selected patients with left main coronary artery disease: the EXCEL trial. EuroIntervention. 2016;12(7):861-872. https://doi.org/10.4244/EIJV12I7A141
- Cain MT, Joyce DL, Szabo A. Reduced morbidity and mortality associated with minimally invasive single-vessel coronary artery bypass compared with conventional sternotomy. Ann Surg. 2023;277(5):e1176-e1183. https://doi.org/10.1097/SLA.0000000000005511
- Dokollari A, Sicouri S, Erten O. Long-term clinical outcomes of robotic-assisted surgical coronary artery revascularisation. EuroIntervention. 2024;20(1):45-55. https://doi.org/10.4244/EIJ-D-23-00373
- Hildick-Smith D, Egred M, Banning A. The European bifurcation club Left Main Coronary Stent study: a randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur Heart J. 2021;42(37):3829-3839. https://doi.org/10.1093/eurheartj/ehab283
- Rigatelli G, Zuin M, Baracca E. Long-term clinical outcomes of isolated ostial left anterior descending disease treatment: ostial stenting versus left main cross-over stenting. Cardiovasc Revasc Med. 2019;20(12):1058-1062. https://doi.org/10.1016/j.carrev.2019.01.030
- Kishi K, Kimura T, Morimoto T. Sirolimus-eluting stent implantation for ostial left anterior descending coronary artery lesions: three-year outcome from the j-Cypher Registry. Circ Cardiovasc Interv. 2011;4(4):362-370. https://doi.org/10.1161/CIRCINTERVENTIONS.111.961904
- Sorm Z, Harrer J, Voborník M, Cermáková E, Vojácek J. Early and long-term results of minimally invasive coronary artery bypass grafting in elderly patients. Kardiol Pol. 2011;69(3):213-218.
- Ushioda R, Hirofuji A, Yoongtong D. Multi-vessel coronary artery grafting: analyzing the minimally invasive approach and its safety. Front Cardiovasc Med. 2024;11():1391881-. https://doi.org/10.3389/fcvm.2024.1391881
- Edwards J, Binongo J, Mullin B. Intensive Care Unit bypass for robotic-assisted single-vessel coronary artery bypass grafting. Ann Thorac Surg. 2023;115(2):511-517. https://doi.org/10.1016/j.athoracsur.2022.06.044
- Sampon F, Ter Woorst J, Dekker L, Akca F. Thoracoscopic-assisted, minimally invasive versus off-pump bypass grafting for single vessel coronary artery disease - A propensity matched analysis. Int J Cardiol. 2024;409():132175-. https://doi.org/10.1016/j.ijcard.2024.132175
- Choi W, Chang HW, Kang SH. Comparison of minimally invasive direct coronary artery bypass and percutaneous coronary intervention using second-generation drug-eluting stents for coronary artery disease-propensity score-matched analysis. Circ J. 2019;83(7):1572-1580. https://doi.org/10.1253/circj.CJ-18-1330
- Etienne PY, D'hoore W, Papadatos S. Five-year follow-up of drug-eluting stents implantation vs minimally invasive direct coronary artery bypass for left anterior descending artery disease: a propensity score analysis. Eur J Cardiothorac Surg. 2013;44(5):884-890. https://doi.org/10.1093/ejcts/ezt137
- Aziz O, Rao C, Panesar SS. Meta-analysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ. 2007;334(7594):617-. https://doi.org/10.1136/bmj.39106.476215.BE
- Patel AJ, Yates MT, Soppa GK. What is the optimal revascularization technique for isolated disease of the left anterior descending artery: minimally invasive direct coronary artery bypass or percutaneous coronary intervention?. Interact Cardiovasc Thorac Surg. 2014;19(1):144-148. https://doi.org/10.1093/icvts/ivu076
- Yang ZK, Hu J, Ding FH, Ni JW, Zhang RY, Shen WF. One-year outcome of single-stent crossover versus accurate ostial stenting for isolated left anterior descending ostial stenosis. Coron Artery Dis. 2022;31(1):e67-e72. https://doi.org/10.1097/MCA.0000000000001071
- Shi H, Hyasat K, Deshmukh T. Optimal percutaneous treatment approach to unprotected ostial left anterior descending artery disease: a meta-analysis and systematic review. Heart Lung Circ. 2024;33(8):1123-1135. https://doi.org/10.1016/j.hlc.2024.02.006
- Çizgici AY, Güner A, Alizade E. Cardiovascular outcomes of complex bifurcation lesions following double kissing crush or nano-crush techniques: the multicenter EVOLUTE-CRUSH V study. Catheter Cardiovasc Interv. 2024;104(2):191-202. https://doi.org/10.1002/ccd.31137
- Uzun F, Güner A, Demirci G. Comparison of long-term outcomes of double kissing crush versus T and minimal protrusion techniques in complex bifurcation lesions: the evolute-crush II registry. Catheter Cardiovasc Interv. 2024;103(4):511-522. https://doi.org/10.1002/ccd.30986
- Güner A, Uzun F, Çizgici AY. Long-term cardiovascular outcomes after mini-crush or T and minimal protrusion techniques in complex bifurcation lesions: the evolute-crush III study. Coron Artery Dis. 2024;35(8):641-649. https://doi.org/10.1097/MCA.0000000000001392
- Uzun F, Güner A, Serin E. Mini-crush or nano-crush stenting technique for complex coronary bifurcation lesions: the multicenter MINANO registry. Can J Cardiol. 2025;():S0828-282X(25)00015-7-. https://doi.org/10.1016/j.cjca.2025.01.008
- Fukui T, Uchimuro T, Takanashi S. EuroSCORE II with SYNTAX score to assess risks of coronary artery bypass grafting outcomes. Eur J Cardiothorac Surg. 2015;47(1):66-71. https://doi.org/10.1093/ejcts/ezu045


