2Department of Cardiology, Muş State Hospital, Muş, Türkiye
3Department of Cardiology, Başakşehir Çam and Sakura City Hospital, İstanbul, Türkiye
Abstract
Background: The precise etiology of hypoplasia of the posterior mitral valve leaflet (PMVL) remains incompletely elucidated; however, it has been hypothesized to stem from genetic mutations occurring during fetal development. Herein, we present the anatomical characteristics of the mitral valve and associated cardiac pathologies in patients with hypoplastic PMVL.
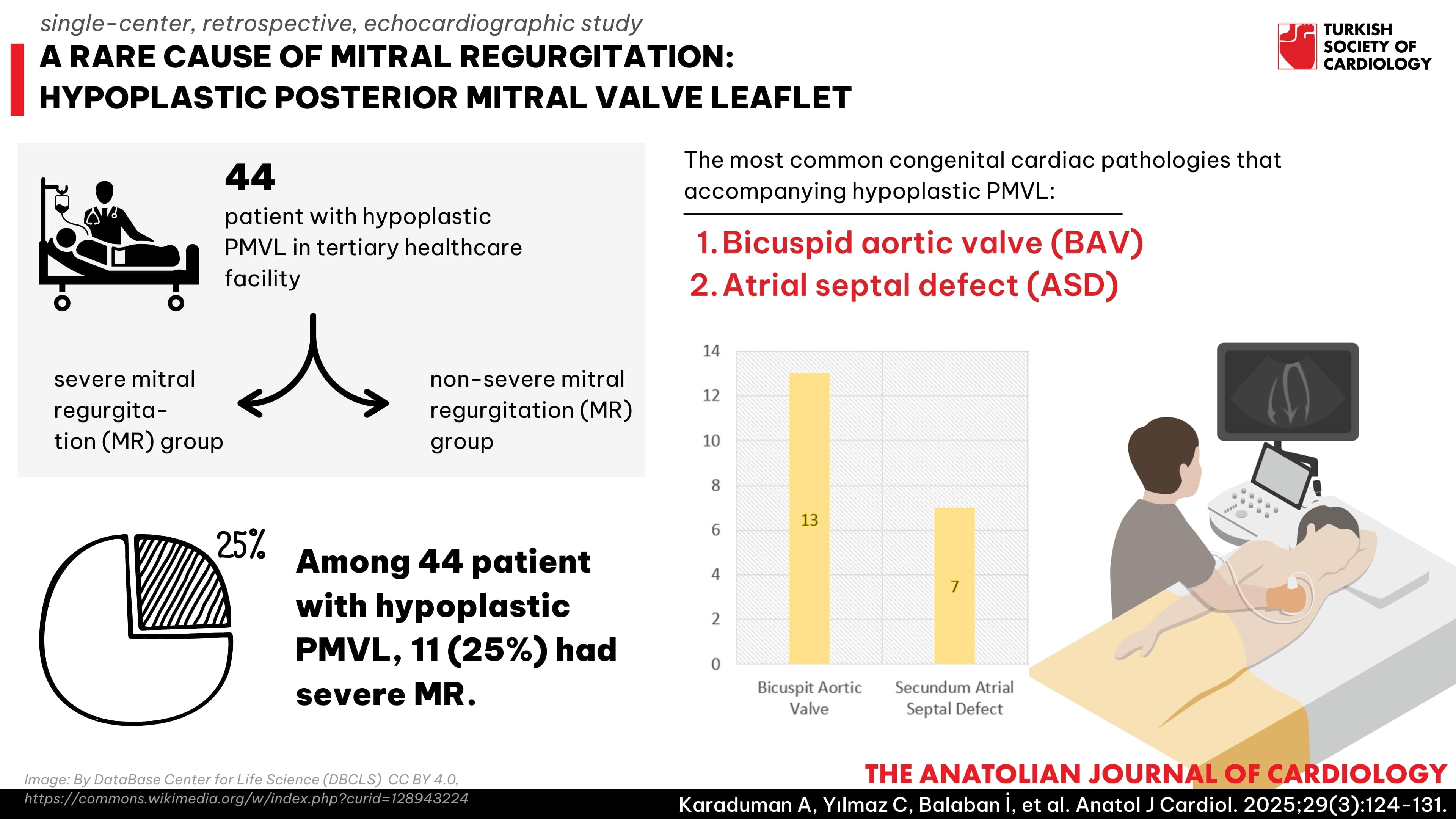
Methods: This single-center retrospective study involved patients who presented between 2015 and 2021 at a tertiary healthcare facility. Among the cohort, 44 individuals had hypoplastic PMVL and were divided into 2 groups: those with severe mitral regurgitation (MR) and those with non-severe MR.
Results: Among the patients, 11 (25%) had severe MR. The median lengths for the PMVL was 5 mm (5-6). Moreover, 10 patients had concomitant muscular formation. We found that 13 patients had bicuspid aortic valve (BAV), while the second most common concomitant cardiac congenital pathology was secundum atrial septal defect (ASD) in 7 patients. The anterior mitral leaflet (AML) length (P = .007), AML prolapse (P < .001), and A2P2 distance (P = .008) were higher in the group with severe MR. In addition, muscular formation was more common in patients with hypoplastic PMVL with severe MR (P < .001).
Conclusion: Hypoplastic PMVL is a rare but significant anomaly that causes MR. While it can coexist with numerous congenital conditions, the most frequent associations include BAV and, secondly, ASD. Severe MR is particularly observed in cases accompanied by dilated mitral annulus, AML prolapse, and muscular formation.
Graphical Abstract
Highlights
- Hypoplastic posterior mitral valve leaflet is a rare but significant anomaly that causes mitral regurgitation.
- Although it can coexist with various congenital conditions, it is most commonly associated with bicuspid aortic valve and atrial septal defect.
- Severe mitral valve regurgitation is particularly observed in cases accompanied by dilated mitral annulus, anterior mitral leaflet prolapse, and muscular formation.
Introduction
Mitral valve complex comprises leaflets, chordae tendineae, papillary muscles, and commissures. Mitral valve regurgitation (MR) arises from deficiencies in any of these anatomical components. Mitral leaflet defects encompass cleft leaflet, accessory mitral valve, aplasia, or hypoplasia of the leaflets.1 Severe hypoplasia and posterior mitral valve leaflet (PMVL) agenesis (aplasia) are rare causes of MR in adults.
Descriptions of unileaflet mitral valves (partial or complete leaflet agenesis/hypoplasia) are exceedingly rare, mostly confined to case reports.2-
Method
Study Population
Patients who presented to our hospital between 2015 and 2021 were included in the study. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) data from patients attending the Echocardiography Laboratory during this period were retrospectively reviewed. Within this cohort, 44 individuals were identified with hypoplastic PMVL anomalies. Both symptomatic and asymptomatic patients with MR were included in the study. All the patients underwent both TTE and TEE. The echocardiograms performed during the specified years were retrospectively analyzed, and detailed echocardiographic assessments were carried out at the time of initial diagnosis for those with hypoplastic PMVL, without the need for patient recall for further evaluations.
Clinical, epidemiological, and laboratory data were collected from electronic health records at our institution. Hypertension (HT) was determined by a prior diagnosis, systolic blood pressure (BP) above 140 mm/Hg, or diastolic BP above 90 mm Hg. Diabetes mellitus (DM) was diagnosed based on patient history, fasting plasma glucose levels exceeding 126 mg/dL, random plasma glucose levels surpassing 200 mg/dL, HbA1c values greater than 6.5%, or the use of anti-diabetic medications. This study was performed in line with the principles of the Declaration of Helsinki. Approval granted by the Ethics Committee of our hospital. The research presented in this manuscript was developed without the utilization of artificial intelligence.
Echocardiography
Transthoracic echocardiography examinations were performed utilizing a Philips S5 transducer (Philips Healthcare, Andover, MA, USA), while two-dimensional (2D) and three-dimensional (3D) multiplane TEE were conducted using a Philips IE33 xMatrix system with an X7-2t multiplane probe (Philips Healthcare). Echocardiographic data were comprehensively analyzed offline using commercially available workstation software. The cardiac measurements were evaluated by 2 experienced cardiologists specializing in imaging, and the mean value of the 2 measurements was calculated. Both the intraobserver and interobserver variability were determined to be less than 5%, based on repeated measurements in 10 randomly selected patients. The average of the 2 cardiologists’ measurements was used to minimize variability, and we acknowledge that this level of variability could still impact the precision of our findings.
The midesophageal bicommissural view on TEE was utilized to assess mitral valve scallops using the X-plane mode. In the study by Abazid et al7 in 2023, it was discovered that 3D TEE exhibited significant concordance with the commissural-biplane approach. Similarly, we conducted our mitral valve scallop measurements using this method. The commissural-biplane approach involved acquiring the bicommissural view and simultaneously capturing biplane images of the medial (A3), middle (A2), and lateral (A1) aspects of the anterior mitral valve. The posterior mitral valve leaflet length was measured in the midesophageal long-axis view on TEE but was not evaluated as separate scallops (
In all patients, pulmonary veins were evaluated using TEE. Additional examinations were not performed in patients without clinical or echocardiographic suspicion of anomalies. Such examinations were only conducted in cases where there was suspicion of partial anomalous pulmonary venous return (PAPVR).
Although 3D echocardiography was performed on many of the patients, it was not applied to all of them. Specifically, 3D echocardiography was conducted in 30 out of the 44 patients, while in the remaining 14 patients, only 2D echocardiography was used. Unfortunately, some patients have since passed away, undergone surgical valve replacement, or were lost to follow-up, making it impossible to perform additional evaluations.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) version 25 was used for statistical analysis (IBM Corp., Armonk, New York, USA). The normal distribution of numerical variables was assessed using the Shapiro–Wilk normality test, histogram, skewness, kurtosis, P–P plot, and Q–Q plot tests. Descriptive statistics were presented as number of units (n), percentage (%), mean ± standard deviation, median, and interquartile range values. The homogeneity of variances was evaluated using the Levene test. Two-group comparisons for numerical variables were performed using the independent samples
Results
This study included 44 patients with hypoplastic PMVL. Baseline clinical and echocardiographic characteristics of the patients are shown in
The characteristics of patients with and without severe MR are presented in
Discussion
Although hypoplastic PMVL has been described in prior case reports and case series, this study is, to the best of our knowledge, among the first to specifically focus on this group of patients. In the present study, the PMVL characteristics and concomitant congenital cardiac pathologies in patients with hypoplastic PMVL were demonstrated.
The study conducted by Rusted et al8 on 50 cadavers revealed that the average length of the posterior leaflet of the mitral valve was 1.2 cm in females, with a range between 0.7 and 2.4 cm. In males, the average length was slightly longer at 1.3 cm, with a range spanning from 0.8 to 1.8 cm. The free edge of the posterior leaflet is often divided into 3 or more scallops or described as lateral, middle, and medial or segments P1, P2, and P3.9 Shree et al10 conducted a study on 50 cadaveric hearts and demonstrated the presence of a variable number of scallops in the posterior leaflet, ranging from single, double, triple, quadruple, or tetra–scalloped, with the most notable configurations being 6 or hexa–scalloped. Although 3 scallops are most common, the scallops are not equal in size. Ranganathan et al11 found the P2 scallop to be larger in the majority of hearts. However, although there are autopsy studies regarding the lengths of mitral valve scallops, there is no large-scale echocardiographic study determining the lengths of normal mitral valve scallops. In our study, we were also unable to evaluate the PMVL as separate scallops in patients with hypoplastic PMVL due to insufficient tissue.
The absence of the PMVL typically leads to fetal demise in utero. Hypoplasia of the PMVL has been linked with stenosis of the supravalvular mitral ring.12 Additionally, individuals with this anomaly may tolerate the condition into adulthood, with the gradual development of MR stemming from annular dilation. The prognosis for asymptomatic patients with hypoplastic mitral valves is uncertain. There is a potential risk of worsening MR, primarily due to annular dilation, which may lead to increased morbidity and mortality.6 In this study, annular dilation of the mitral valve was found to be potentially associated with severe MR. The A2P2 distance was greater in patients with severe MR. Furthermore, AML prolapse, which may occur as a compensatory mechanism for annular dilation, was more commonly observed in the severe MR group. It was also found herein that the severe MR group had a longer AML.
Furthermore, in our study, differences were observed in left atrial diameter (LAD), left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD) between individuals with severe MR and those without. However, it is possible that this difference may also be influenced by the duration of exposure to severe MR. Nevertheless, we do not have data related to this exposure duration. Additional studies are required to address this issue.
Distinguishing hypoplastic PMVL from rheumatic mitral valve disease is crucial. In hypoplastic PMVL cases, thickened and restricted leaflets may be observed, mimicking the appearance of end-stage rheumatic valve disease. However, in cases where rheumatic factors are implicated, it typically takes several decades for severe stenotic valvulopathy to develop after recurrent episodes of carditis.
Muscular formation is a cardiac pathology accompanying hypoplastic PMVL patients.13-
There are various other cardiac anomalies reported in hypoplastic PMVL patients. In the patients with hypoplastic PMVL in the present study, the most common accompanying congenital cardiac pathology was aortic valve diseases. Among aortic valve diseases, BAV is the most common, as demonstrated in many cases in the literature.5,
In patients with hypoplastic PMVL, the most significant valve issue is severe mitral insufficiency. In the literature, valve replacement is commonly performed in many hypoplastic PMVL patients with severe MR.3,
Currently, no genetic analyses are available for the patients included in our follow-up. However, we recognize the importance of genetic studies in understanding the potential hereditary factors and mutations associated with hypoplastic PMVL. This area represents a promising direction for future research. Although all of our patients underwent TTE and TEE, cardiac magnetic resonance imaging (MRI) could provide a more comprehensive assessment of hypoplastic PMVL and associated cardiac pathologies. Since no studies have been conducted using cardiac MRI for this purpose in the literature, this represents an area for potential future investigation.
Study Limitations
This study had several limitations. First, being a single-center cross-sectional observational study, there was the possibility of selection bias. Therefore, the results should be validated in larger, more diverse populations. Second, our center serves as a reference hospital due to its status as a high-volume cardiac center. Therefore, the incidence of hypoplastic PMVL cannot be extrapolated to the general population, and our patient selection is not consecutive. Prospective and multicenter large-volume studies are imperative to elucidate the anatomical characteristics of the mitral valve in patients with hypoplastic PMVL and to understand the associated cardiac pathologies within this patient cohort. Another limitation of our study is the potential impact of interobserver and intraobserver variability on the precision of cardiac measurements, despite efforts to minimize it by averaging the measurements taken by 2 experienced cardiologists. An additional limitation is the lack of cardiac MRI, which could provide a more comprehensive assessment of muscular formation.
Conclusion
In conclusion, hypoplastic PMVL is a rare but significant anomaly that causes mitral regurgitation. While it can coexist with numerous congenital conditions, the most frequent associations include BAV and, secondly, ASD. Severe MR is particularly observed in cases accompanied by dilated mitral annulus, AML prolapse, and muscular formation.
Footnotes
References
- Oosthoek PW, Wenink AC, Wisse LJ, Gittenberger-de Groot AC. Development of the papillary muscles of the mitral valve: morphogenetic background of parachute-like asymmetric mitral valves and other mitral valve anomalies. J Thorac Cardiovasc Surg. 1998;116(1):36-46.
- Yazdan-Ashoori P, Rohani A, Mulji AS, Van Spall HG. Hypoplasia of the posterior mitral valve leaflet detected in late adulthood. Eur Heart J. 2015;36(7):456-.
- Bacich D, Braggion G, Faggian G. Hypoplasia of the posterior mitral leaflet: a rare cause of mitral regurgitation in adulthood. Echocardiography. 2017;34(6):949-950.
- Candan O, Guler A, Aung SM, Gecmen C, Karabay CY, Yildiz M. Uni-leaflet mitral valve. Eur J Echocardiogr. 2011;12(8):640-.
- Bär H, Siegmund A, Wolf D, Hardt S, Katus HA, Mereles D. Prevalence of asymptomatic mitral valve malformations. Clin Res Cardiol. 2009;98(5):305-309.
- Kanagala P, Baker S, Green L, Houghton AR. Functionally uni-leaflet mitral valves in a family: a case series. Eur J Echocardiogr. 2010;11(7):E27-.
- Abazid RM, Frost A, Manian U. Diagnostic accuracy of transesophageal echocardiographic commissural-biplane approach in identifying mitral valve anatomy. J Am Soc Echocardiogr. 2023;36(9):956-962.
- Rusted IE, Scheifley CH, Edwards JE. Studies of the mitral valve. I. Anatomic features of the normal mitral valve and associated structures. Circulation. 1952;6(6):825-831.
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg. 1983;86(3):323-337.
- Shree B, Singla RK, Soni S. Posterior leaflet of mitral valve-is it really tri-scalloped? -A morphological and morphometric study in north indian cadaveric hearts. Adv Biomed Res. 2023;12():229-.
- Ranganathan N, Lam JH, Wigle ED, Silver MD. Morphology of the human mitral valve. II. The value leaflets. Circulation. 1970;41(3):459-467.
- Zhang J, Sun F, Ren W, Xiao Y, Zhan Y. Hypoplasia of the posterior mitral leaflet concurrent with a supravalvular mitral ring: a rare cause of congenital mitral stenosis. J Ultrasound Med. 2014;33(4):736-738.
- Pourafkari L, Baghbani-Oskouei A, Toufan M, Ghaffari S, Nader ND. Hypoplastic posterior mitral valve leaflet: A case report and review of the literature. Echocardiography. 2018;35(7):1052-1055.
- Karabay CY, Guler A, Aung SM. An incomplete heart with incomplete lungs. Echocardiography. 2012;29(9):E247-E249.
- Kalangos A, Oberhansli I, Baldovinos A, Beghetti M, Friedli B, Faidutti B. Hypoplasia of the posterior leaflet as a rare cause of congenital mitral insufficiency. J Card Surg. 1997;12(5):339-342.
- Saura D, Oliva MJ, Sanchez-Galian MJ. Real-time three-dimensional transesophageal echocardiographic evaluation of the association of bicuspid aortic valve and mitral posterior leaflet hypoplasia. Int J Cardiol. 2015;195():334-335.
- Fazlinezhad A, Alvandi Azari M, Bigdellu L. Severe hypoplasia of posterior mitral valve leaflet presented with atypical chest pain: a case report. Razavi Int J Med. 2017;5(1):e41501-.
- Heper G, Yetkin E, Senen K. Absence of posterior mitral leaflet with secundum atrial septal defect. Ann Thorac Surg. 2010;90(6):2055-2057.
- Bhardwaj A, Pillai AA, Balaguru S. A case of hypoplastic posterior mitral leaflet-related severe mitral regurgitation associated with ostium secundum atrial septal defect. Eur Heart J. 2022;43(36):3497-.
- de Agustin JA, Gomez de Diego JJ, Garcia-Fernandez MA. Severe hypoplasia of the posterior mitral leaflet: a rare cause of congenital mitral regurgitation assessed by three-dimensional transesophageal echocardiography. Int J Cardiol. 2014;177(3):e131-e132.
- Parato VM, Masia SL. Hypoplasia or absence of posterior leaflet: A rare congenital anomaly of the mitral valve in adulthood - case series. J Cardiovasc Echogr. 2018;28(1):45-47.
- Joshi V, Laurie K, Skoyles J, Richens D. Severe mitral regurgitation secondary to atresia of the posterior mitral valve leaflet in the adult: is repair always best practice?. Thorac Cardiovasc Surg Rep. 2015;4(1):34-36.
- Caciolli S, Gelsomino S, Fradella G, Bevilacqua S, Favilli S, Gensini GF. Severe hypoplasia of the posterior mitral leaflet. Ann Thorac Surg. 2008;86(6):1978-1979.
- Stojanovic I, Vukovic P, Boskovic S, Vuk LL, Korac NS. Repair of hammock mitral valve with hypoplastic posterior leaflet in an adult. J Heart Valve Dis. 2010;19(6):803-805.
- Gupta A, Gharde P, Kumar AS. Anterior mitral leaflet length: predictor for mitral valve repair in a rheumatic population. Ann Thorac Surg. 2010;90(6):1930-1933.


