2Department of Infectious Diseases and Clinical Microbiology, Ege University Hospital, İzmir, Türkiye
3Department of Infectious Diseases and Clinical Microbiology, İzmir Bozyaka Training and Research Hospital, İzmir, Türkiye
4Department of Infectious Diseases and Clinical Microbiology, Uludağ University Hospital, Bursa, Türkiye
5Department of Infectious Diseases and Clinical Microbiology, Kartal Dr. Lütfi Kırdar City Hospital, İstanbul, Türkiye
6Department of Infectious Diseases and Clinical Microbiology, Ondokuz Mayıs University Hospital, Samsun, Türkiye
7Department of Infectious Diseases and Clinical Microbiology, Haydarpaşa Numune Training and Research Hospital, İstanbul, Türkiye
8Department of Infectious Diseases and Clinical Microbiology, Akdeniz University Hospital, Antalya, Türkiye
9Department of Infectious Diseases and Clinical Microbiology, Bilkent City Hospital, Ankara, Türkiye
10Department of Infectious Diseases and Clinical Microbiology, Ümraniye Training and Research Hospital, İstanbul, Türkiye
11Department of Infectious Diseases and Clinical Microbiology, Gaziantep University Hospital, Gaziantep, Türkiye
12Department of Infectious Diseases and Clinical Microbiology, İstanbul University Hospital, İstanbul, Türkiye
13Department of Infectious Diseases and Clinical Microbiology, Katip Çelebi University Atatürk Training and Research Hospital, İzmir, Türkiye
14Department of Infectious Diseases and Clinical Microbiology, Prof. Dr. Cemil Taşçıoğlu City Hospital, İstanbul, Türkiye
15Department of Infectious Diseases and Clinical Microbiology, Ankara University Hospital, Ankara, Türkiye
16Department of Infectious Diseases and Clinical Microbiology, İstanbul Training and Research Hospital, İstanbul, Türkiye
17Department of Infectious Diseases and Clinical Microbiology, Dokuz Eylül University Hospital, İzmir, Türkiye
18Department of Infectious Diseases and Clinical Microbiology, Pamukkale University Hospital, Denizli, Türkiye
19Department of Infectious Diseases and Clinical Microbiology, Ankara Training and Research Hospital, Ankara, Türkiye
20MSD Türkiye, and HIV-TR Study Group
Abstract
Background: Cardiovascular disease (CVD) is a major cause of mortality among people living with HIV (PLWH). We aimed to assess the prevalence of diagnosed CVD and the risk of CVD among PLWH using 5 different tools.
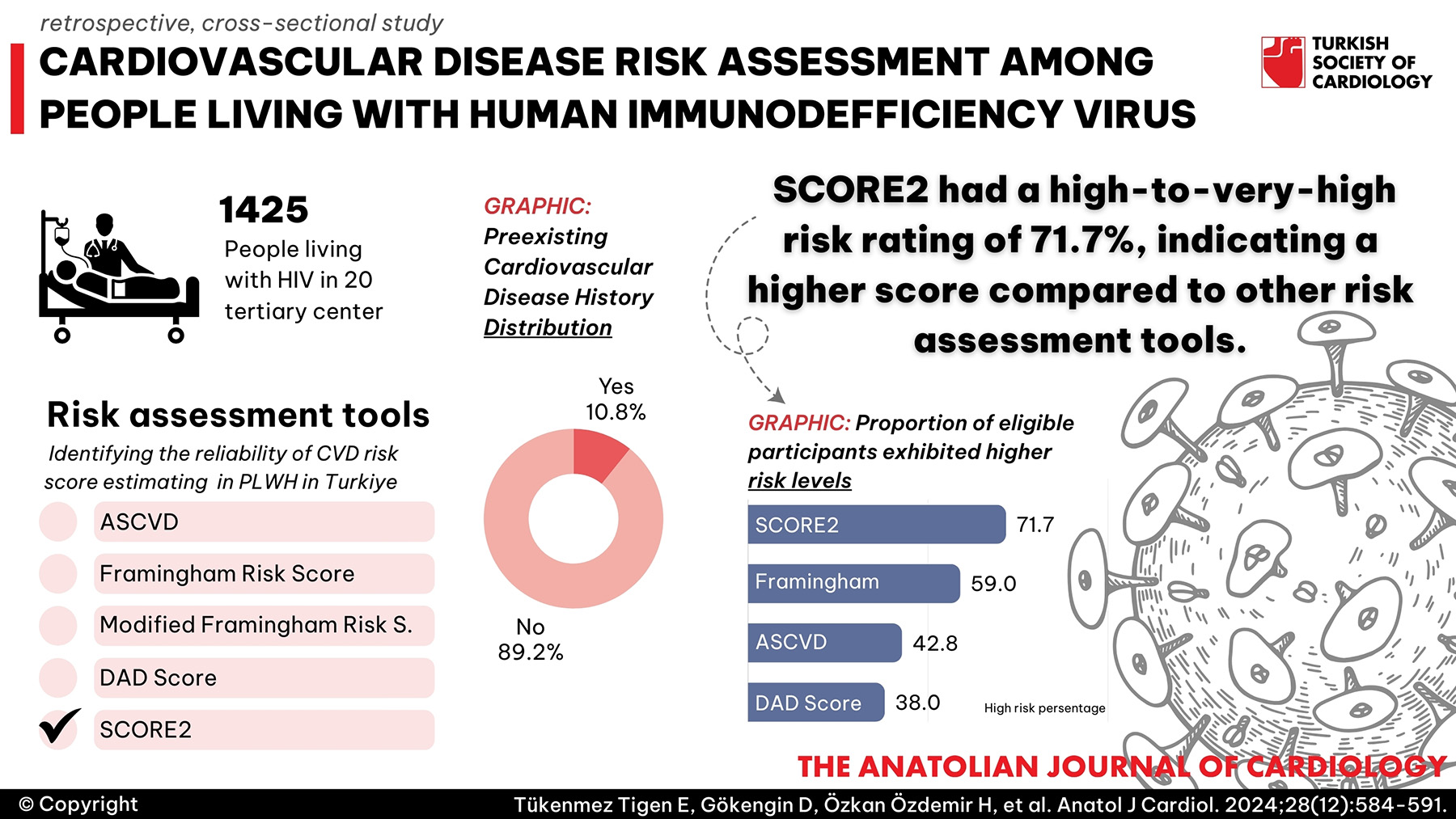
Methods: This retrospective, cross-sectional study was conducted in 20 tertiary centers in Türkiye between October 2021 and March 2022, among 1425 PLWH aged 40-75 years. About 82.7% were male, with a median age of 51. Web-based tools for each score were used for CVD risk calculations.
Results: Of 1425 PLWH enrolled, 10.8% had confirmed CVD, and 1132 had their risk scores evaluated. Of those participants, 42.8% had a higher risk of CVD (10-year risk of atherosclerotic CVD risk score (ASCVD) above 7.5%), and according to the European Society of Cardiology systemic coronary risk evaluation 2 (SCORE2), 71.7% had a high- to very high-risk rate. The agreement between various CVD risk tools varied, with Framingham heart study risk score (FRS), modified FRS, data collection on adverse effects of anti-HIV drugs (DAD), and SCORE2 for high-risk countries showing overall agreement rates of 82%, 94%, 91%, and 36%, respectively, compared to ASCVD. According to the 2021 European and 2019 American Cardiology guidelines, 75.3% and 47.1% of PLWH would be eligible for lipid-lowering agents, respectively.
Conclusion: The diagnosed CVD prevalence highlighted the importance of monitoring cardiovascular health and comorbidities in this population. SCORE2 identified a greater number of individuals at high/very high risk compared to other prediction tools. The implementation of CVD prevention through lipid-lowering therapy was far from desired levels in our cohort.
Graphical Abstract
Highlights
- Cardiovascular disease (CVD) is a major cause of mortality among people living with HIV (PLWH).
- Identifying the most reliable risk score for estimating CVD risk in PLWH is crucial.
- SCORE2 had a high-to-very-high risk rating of 71.7%, indicating a higher score compared to other risk assessment tools.
- According to the 2021 European and 2019 American Cardiology guidelines, 75.3% and 47.1% of PLWH would be eligible for lipid-lowering agents.
Introduction
People living with human immunodeficiency virus (PLWH) share similar cardiovascular risk factors with the general population, and HIV infection itself is considered a risk-enhancing factor for cardiovascular disease (CVD).1,
Multiple CVD risk prediction algorithms with different endpoints are available; however, the majority are developed for the general population and may not fully capture the unique risk profile of PLWH. The data collection on adverse effects of anti-HIV drugs (DAD) score is the only tool specifically developed for PLWH that incorporates antiretroviral therapy (indinavir, lopinavir/ritonavir, and abacavir) exposure to enhance risk assessment.6 A reduced version of the DAD score (DADr), which considers only the CD4+ T cell count and excludes antiretroviral treatments, is also available and was updated in 2016.7 However, the prevalent practice leans on risk prediction models designed for the general population when recommending preventive therapies, including lipid-lowering medications, for PLWH. These models notably include the atherosclerotic CVD risk score8 (ASCVD), the Framingham heart study risk score9 (FRS), and the systemic coronary risk evaluation (SCORE) developed by the European Society of Cardiology.10 The SCORE evaluates the 10-year risk of a fatal atherosclerotic event (e.g., stroke, aortic aneurysm, myocardial infarction). Its 2021 updated version SCORE2 estimates the total burden of CVD, including non-fatal CVD events, particularly among younger individuals; compared to the SCORE tool, its risk discrimination performance is better and it accounts for the impact of competing risks by non-CVD deaths, a factor overlooked by the SCORE tool.11 SCORE2-older persons (SCORE2-OP), which is a complementary assessment tool, estimates non-fatal and fatal CVD events adjusted for competing risk in healthy people aged >70 years.12 In these tools, the model was recalibrated according to geographical risk regions and the European countries were grouped into 4 risk regions (low, moderate, high, and very high) in compliance with the World Health Organization report on the risk of CVD mortalities. This adjustment provides a benefit for treatment decisions in older individuals and those from high- or very high-risk regions.13 Framingham heart study risk score, ASCVD, and DAD scoring tools have been shown to underestimate the CVD risk in PLWH.3,
Very few studies report on the agreement between the commonly used risk estimation equations among PLWH in Türkiye. Notably, the performance of recalibrated SCORE2 in determining CVD risk in Türkiye's high-risk context remains unexplored. Our primary objective was to assess the prevalence of diagnosed CVD, both overall and categorized by specific components such as myocardial infarction, angina pectoris, ischemic and hemorrhagic stroke, transient ischemic attack, and invasive vascular procedures. Furthermore, we aimed to determine the proportion of patients without a prior CVD diagnosis who fell into different CVD risk categories calculated using 5 distinct risk assessment tools. Secondary objectives included comparing the level of agreement among these risk assessment tools and estimating statin eligibility within this multicenter cohort in Türkiye.
Methods
This is a retrospective cross-sectional observational study of the HIV-TR cohort including 20 hospitals located in 8 different cities of Türkiye. The HIV-TR cohort consists of approximately one-third of PLWH receiving treatment in Türkiye. Demographic, clinical, laboratory, and treatment data were extracted from medical records of the data collection system. The cohort consists of PLWH aged between 40 and 75 years, both with and without CVD diagnoses, who have been receiving antiretroviral therapy (ART) for at least 6 months. Data were collected from consecutive outpatient clinic visits at participating hospitals between October 2019 and October 2021. Patients with less than 6 months of ART usage and those without a follow-up visit in the previous 2 years were excluded. All these data were evaluated between October 2021 and March 2022.
The risk of CVD and eligibility for lipid-lowering therapy were assessed in individuals who had no prior history of CVD and were not currently using lipid-lowering medications. Various web-based tools were used for risk calculations including (ASCVD:
Persons are considered at higher risk if the 10-year CVD risk was ≥20% with FRS;9 2.5 to <7.5% for age under 50; >10% for age 50-69, >15% for age >70 with SCORE2/SCORE2-OP;11,
Based on CVD risk interpretation, the individuals were placed in 2 categories: low/medium and high/very high. Statin eligibility was determined based on the 2019 American College of Cardiology/American Heart Association (ACC/AHA) Cholesterol Management Guidelines and the 2021 ESC guidelines using SCORE2.13,
The study protocol received approval on October 8, 2021 (Approval No: 1151). No artificial intelligence programs were used during the production of this manuscript.
Statistical Analysis
The normality of continuous variables was assessed using Shapiro–Wilk’s test. Descriptive statistics included mean and SD for normally distributed variables, and median with interquartile range (IQR) for non-normally distributed variables. Statistical analyses were performed using MedCalc Statistical Software version 12.7.7 (Ostend, Belgium).18
The agreement between different CVD risk scores was evaluated using Cohen’s kappa (κ) statistics with 95% confidence intervals (CIs). Agreement levels were categorized as follows: poor (κ = 0.20), fair (κ = 0.21-0.40), moderate (κ = 0.41-0.60), substantial (κ = 0.61-0.80), and very good (κ > 0.80).19
Results
A comprehensive analysis was conducted on 1425 PLWH, whose baseline characteristics are detailed in
According to the 2021 European13 and 2019 American Cardiology guidelines,16 75.3% and 47.1% of cohort participants would be eligible for lipid-lowering agents, respectively. Among the 189 individuals taking lipid-lowering medications at baseline (and thus excluded from the main cohort analysis), 18.5% still had high cholesterol levels (total cholesterol >240 mg/dL or LDL >160 mg/dL). Among these patients, the mean LDL (mg/dL) level was 120.5 ± 37.9. Due to the retrospective nature of the study, it was not feasible to ascertain the usage patterns of lipid-lowering agents.
Discussion
To our knowledge, this study represents the first large-scale multicenter investigation in Türkiye evaluating the CVD risk among PLWH. It utilizes various CVD risk scores, including SCORE2, to assess risk and determine eligibility for lipid-lowering treatment in accordance with commonly used guidelines. Additionally, the study aims to assess the prevalence of diagnosed CVD among PLWH.
Several CVD prediction models, initially developed for the general population such as FRS, ASCVD, and SCORE, have been widely utilized and validated in PLWH in high-income countries. In the HIV outpatient study (HOPS) cohort, while FRS accurately estimated the risk of CVD events, the risk was underestimated with ASCVD and DAD.20 Conversely, in an Italian cohort, ASCVD and DAD models exhibited slightly better performance than FRS, with all algorithms demonstrating moderate specificity, sensitivity, and positive predictive values, alongside high negative predictive values.21 In a Dutch cohort, the FRS-CVD, ASCVD, and SCORE-NL models classified 65%, 89%, and 85% of PLWH in the same risk category as the DAD.22 Compared to the DAD model, the weighted kappa statistic indicated low agreement for FRS-CVD (0.27) and moderate agreement for SCORE-NL and ASCVD (0.58 and 0.66, respectively). Notably, FRS tended to overestimate CVD risk in this study.22 However, in validation studies among PLWH, most models tended to underpredict CVD risk, possibly due to the underlying mechanisms of disease.23
If real-world studies assessing a model’s performance (discrimination and calibration) are unavailable, understanding the concordance between commonly used models to identify high-risk individuals may assist clinicians.
In an age-comparable Croatian and Serbian cohort, a high 5-year DAD CVD risk score (>5%) showed substantial agreement with an increased (≥7.5%) 10-year ASCVD risk score (
Several studies have reported suboptimal prescription of statins in PLWH. In an Italian cohort, only 50% of PLWH meeting criteria for statins according to the European AIDS Clinical Society (EACS) guidelines were properly treated.25 A propensity score-matched study comparing statin prescribing gap between PLWH and uninfected patients, determined by the ACC/AHA 2013 Guideline on the Treatment of Blood Cholesterol, revealed significantly lower statin use for PLWH (27.8%) than for matched uninfected patients (40.5%).26 Although improvements in statin initiation augmented with the use of 2013 ACC/AHA guidelines were identified among PLWH from 2001 to 2017 in the North American Collaborative on AIDS Cohort Research and Design (NA-ACCORD), statins are still prescribed to less than half of the eligible population.27 Similarly, earlier studies from Western European countries suggested inadequate recognition and addressing of CVD risk factors in PLWH.28,
Türkiye has one of the highest CVD mortality rates among European countries, with 39.7% of deaths caused by CVDs in 2018.30 A projection for 2030 shows a 1.8-fold increase in CVD-related mortality in women and a 2.3-fold increase in men.30 Additionally, the MI rate is high in people younger than 50 years, with the mean age at the index coronary episode nearly 10 years younger than in the overall European population. 31-
Regarding the results of the assessed baseline characteristics, the hypercholesterolemia rate was higher in our cohort than in the general Turkish population with similar age ranges.37 Additionally, compared to the study of Korten et al40 with a similar, smaller Turkish cohort analyzed between 2016-2017, our study showed a higher prevalence of diabetes (7.8% vs 17%), hypertension (21.6% vs 29.5%), and hyperlipidemia (26.4% vs 34.9%) rates among PLWH, suggesting that cardiometabolic risks may be increasing over time.
Therefore, it is critical to be aware of the underlying CVD risk among PLWH in Türkiye and to monitor risk factors such as lifestyle, prescribed ART agents and their related side effects, dyslipidemia, hypertension, age, and family history to inform early interventions. European AIDS Clinical Society guidelines suggest advising on diet and lifestyle in all PLWH and considering ART modification if the 10-year CVD risk is ≥ 10%.41
While most efforts have been directed towards recognizing high CVD risk using several algorithms, Zanni and colleagues showed that using coronary computed tomographic angiography in PLWH without known CVD, only 26% of individuals with coronary plaque with high-risk morphology would meet the criteria for statin therapy according to the 2013 ACC-AHA guidelines.42 Further reduction of cardiovascular risk in PLWH assessed as having low to moderate risk based on a traditional disease risk assessment tool was addressed in the Randomized Trail to Prevent Vascular Events in HIV (REPRIEVE) study.43 PLWH who received pitavastatin had a lower risk (4.81 per 1000 person-years) than those who received placebo (7.32 per 1000 person-years) over 5 years. The benefit of pitavastatin in PLWH was beyond its LDL-cholesterol lowering effects, suggesting a beneficial effect on systemic inflammation. While more evidence is needed to expand statin prescription recommendations to PLWH with low-to-intermediate CVD risk, we need to improve recognition and treatment of those with higher risk. In our study, cholesterol levels were still high (total cholesterol >240 mg/dL or LDL >160 mg/dL) in 18.5% of people taking lipid-lowering medication.
None of the prediction models examined in our study have been assessed for discrimination and calibration performance in a longitudinal cohort in Türkiye. However, it is highly likely that PLWH in Türkiye have worse demographic and comorbidity profiles associated with an increase in CVD risk than populations in countries where prediction models have been developed originally. SCORE-2 placed a greater number of individuals in high or very high-risk strata than other prediction tools. While awaiting a real-world validation study, utilizing SCORE2 for CVD screening in PLWH in Türkiye appears reasonable. Clinicians should be informed about the importance of interventions such as lipid-lowering therapies, lifestyle changes, and treatment optimization in their patient populations.
Study Limitations
Our study has several limitations. First, this was a retrospective study. Therefore, crucial information regarding the longitudinal change in CVD risk over time or the incidence of major adverse cardiovascular events within this cohort is unavailable. Second, the study period overlapped with the COVID-19 pandemic, resulting in restricted hospital admissions for PLWH in certain centers at the study's commencement. Despite these limitations, our findings underscore the importance of close monitoring of CVD risk and identification and treatment of appropriate candidates for statin therapy among PLWH in Türkiye.
Conclusion
Our study assessed CVD prevalence and risk among PLWH in Türkiye using 5 different CVD risk-prediction scores. We observed moderate agreement among the algorithms, except for SCORE2, which showed higher risk in 71.7% of PLWH. Further research is needed to determine whether these scores accurately estimate risk at the population level. The diagnosed CVD prevalence was 10.8%, highlighting the importance of monitoring cardiovascular health, comorbidities, and BMI in this population. Our study also showed important gaps in statin use according to the current guidelines, indicating areas for improvement in patient care. Lifestyle changes, reevaluating antiretroviral treatment, and initiating or optimizing lipid-lowering agents hold promise for enhancing patient outcomes and merit additional investigation.
Footnotes
All authors have reviewed the article, contributed to the analysis and/or interpretation of the data, and provided input. All authors read and approved the final version of the article.
Y.E., T.K., and P.G. are full-time employees of MSD Türkiye.
References
- Aberg JA. Cardiovascular complications in HIV management: past, present, and future. J Acquir Immune Defic Syndr. 2009;50(1):54-64. https://doi.org/10.1097/QAI.0b013e31818ceaa4
- Dubé MP, Cadden JJ. Lipid metabolism in treated HIV Infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):429-442. https://doi.org/10.1016/j.beem.2011.04.004
- Shah ASV, Stelzle D, Lee KK. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100-1112. https://doi.org/10.1161/CIRCULATIONAHA.117.033369
- Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30(10):1495-1509. https://doi.org/10.1097/QAD.0000000000001109
- Lundgren JD, Babiker AG, Gordin F. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795-807. https://doi.org/10.1056/NEJMoa1506816
- Friis-Møller N, Thiébaut R, Reiss P. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491-501. https://doi.org/10.1097/HJR.0b013e328336a150
- Friis-Møller N, Ryom L, Smith C. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214-223. https://doi.org/10.1177/2047487315579291
- Goff DC, Lloyd-Jones DM, Bennett G. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73. https://doi.org/10.1161/01.cir.0000437741.48606.98
- D'Agostino RB, Vasan RS, Pencina MJ. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579
- Conroy RM, Pyörälä K, Fitzgerald AP. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987-1003. https://doi.org/10.1016/s0195-668x(03)00114-3
- . SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42(25):2439-2454. https://doi.org/10.1093/eurheartj/ehab309
- . SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J. 2021;42(25):2455-2467. https://doi.org/10.1093/eurheartj/ehab312
- Visseren FLJ, Mach F, Smulders YM. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337. https://doi.org/10.1093/eurheartj/ehab484
- Delabays B, Cavassini M, Damas J. Cardiovascular risk assessment in people living with HIV compared to the general population. Eur J Prev Cardiol. 2022;29(4):689-699. https://doi.org/10.1093/eurjpc/zwab201
- . Classification and diagnosis of diabetes. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
- Arnett DK, Blumenthal RS, Albert MA. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;140(11):e596-e646. https://doi.org/10.1161/CIR.0000000000000678
- Mach F, Baigent C, Catapano AL. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. https://doi.org/10.1093/eurheartj/ehz455
- Schoonjans F. . MedCalc Statistical Software - Free Trial Available. ;():-. https://www.medcalc.org/.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. https://doi.org/10.2307/2529310
- Thompson-Paul AM, Lichtenstein KA, Armon C. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis. 2016;63(11):1508-1516. https://doi.org/10.1093/cid/ciw615
- Raggi P, De Francesco D, Manicardi M. Prediction of hard cardiovascular events in HIV patients. J Antimicrob Chemother. 2016;71(12):3515-3518. https://doi.org/10.1093/jac/dkw346
- Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289-297. https://doi.org/10.1111/hiv.12300
- Achhra AC, Lyass A, Borowsky L. Assessing cardiovascular risk in people living with HIV: current tools and limitations. Curr HIV AIDS Rep. 2021;18(4):271-279. https://doi.org/10.1007/s11904-021-00567-w
- Begovac J, Dragović G, Višković K. Comparison of four international cardiovascular disease prediction models and the prevalence of eligibility for lipid lowering therapy in HIV infected patients on antiretroviral therapy. Croat Med J. 2015;56(1):14-23. https://doi.org/10.3325/cmj.2015.56.14
- De Socio GV, Ricci E, Parruti G. Statins and Aspirin use in HIV-infected people: gap between European AIDS Clinical Society guidelines and clinical practice: the results from HIV-HY study. Infection. 2016;44(5):589-597. https://doi.org/10.1007/s15010-016-0893-z
- Pincus KJ, Blackman AL, Suen SY. Statin gap in patients living with HIV: assessing dose appropriateness. HIV Med. 2021;22(10):917-923. https://doi.org/10.1111/hiv.13150
- Coburn SB, Lang R, Zhang J. Statins utilization in adults with HIV: the treatment gap and predictors of statin initiation. J Acquir Immune Defic Syndr. 2022;91(5):469-478. https://doi.org/10.1097/QAI.0000000000003083
- Law MG, Friis-Møller N, El-Sadr WM. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7(4):218-230. https://doi.org/10.1111/j.1468-1293.2006.00362.x
- Mueller MC, Usadel S, Kern WV, Zirlik A, Zhou Q. Proportion of patients eligible for statin therapy substantially varies between different cardiovascular disease risk calculators and guidelines used. Int J STD AIDS. 2021;32(13):1188-1195. https://doi.org/10.1177/09564624211029392
- Kayikcioğlu M, Oto A. Control and management of cardiovascular disease in Turkey. Circulation. 2020;141(1):7-9. https://doi.org/10.1161/CIRCULATIONAHA.119.037606
- Tokgözoğlu L, Kaya EB, Erol C, Ergene O. EUROASPIRE III: a comparison between Turkey and Europe. Turk Kardiyol Dern Ars. 2010;38(3):164-172.
- Erol MK, Kayıkçıoğlu M, Kılıçkap M. Baseline clinical characteristics and patient profile of the TURKMI registry: results of a nation-wide acute myocardial infarction registry in Turkey. Anatol J Cardiol. 2020;24(1):43-53. https://doi.org/10.14744/AnatolJCardiol.2020.69696
- Tokgözoğlu L, Kayıkçıoğlu M, Altay S. EUROASPIRE-IV: European Society of Cardiology study of lifestyle, risk factors, and treatment approaches in patients with coronary artery disease: data from Turkey. Turk Kardiyol Dern Ars. 2017;45(2):134-144. https://doi.org/10.5543/tkda.2016.82352
- Timmis A, Townsend N, Gale CP. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12-85. https://doi.org/10.1093/eurheartj/ehz859
- Özer N, Kılıçkap M, Tokgözoğlu L. Data on smoking in Turkey: systematic review, meta-analysis and meta-regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46(7):602-612. https://doi.org/10.5543/tkda.2018.85349
- Ural D, Kılıçkap M, Göksülük H. Data on prevalence of obesity and waist circumference in Turkey: systematic review, meta-analysis and meta regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46(7):577-590. https://doi.org/10.5543/tkda.2018.62200
- Kayıkçıoğlu M, Tokgözoğlu L, Kılıçkap M. Data on prevalence of dyslipidemia and lipid values in Turkey: systematic review and meta-analysis of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46(7):556-574. https://doi.org/10.5543/tkda.2018.23450
- Kılıçkap M, Barçın C, Göksülük H. Data on prevalence of hypertension and blood pressure in Turkey: systematic review, meta-analysis and meta-regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars. 2018;46(7):525-545. https://doi.org/10.5543/tkda.2018.15679
- Yılmaz MB, Kılıçkap M, Abacı A. Temporal changes in the epidemiology of diabetes mellitus in Turkey: a systematic review and meta-analysis. Turk Kardiyol Dern Ars. 2018;46(7):546-555. https://doi.org/10.5543/tkda.2018.88225
- Korten V, Gökengin D, Yildirmak T. Comparison of risk category predictions of Framingham Risk Score (FRS), atherosclerotic cardiovascular disease risk score (ASCVD), systematic coronary risk evaluation (SCORE) and data collection on adverse events of anti-HIV drugs (D:A:D) in HIV infected patients. Open Forum Infect Dis. 2017;4(suppl 1):S215-. https://doi.org/10.1093/ofid/ofx163.432
- . European AIDS clinical society. EACS Guideline version 12.0. . 2023;():-. https://www.eacsociety.org/media/guidelines-12.0.pdf
- Zanni MV, Fitch KV, Feldpausch M. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014;28(14):2061-2070. https://doi.org/10.1097/QAD.0000000000000360
- Grinspoon SK, Fitch KV, Zanni MV. Pitavastatin to prevent cardiovascular disease in HIV infection. N Engl J Med. 2023;389(8):687-699. https://doi.org/10.1056/NEJMoa2304146


