2Department of Cardiothoracic Surgery Research, Lankenau Institute for Medical Research, Wynnewood, Pennsylvania, USA
3Department of Interventional Cardiology, Lankenau Heart Institute, Main Line Health Wynnewood, Pennsylvania, USA
4Department of Cardiothoracic Surgery, Lankenau Heart Institute, Main Line Health Wynnewood, Pennsylvania, USA;Department of Cardiothoracic Surgery Research, Lankenau Institute for Medical Research, Wynnewood, Pennsylvania, USA
Abstract
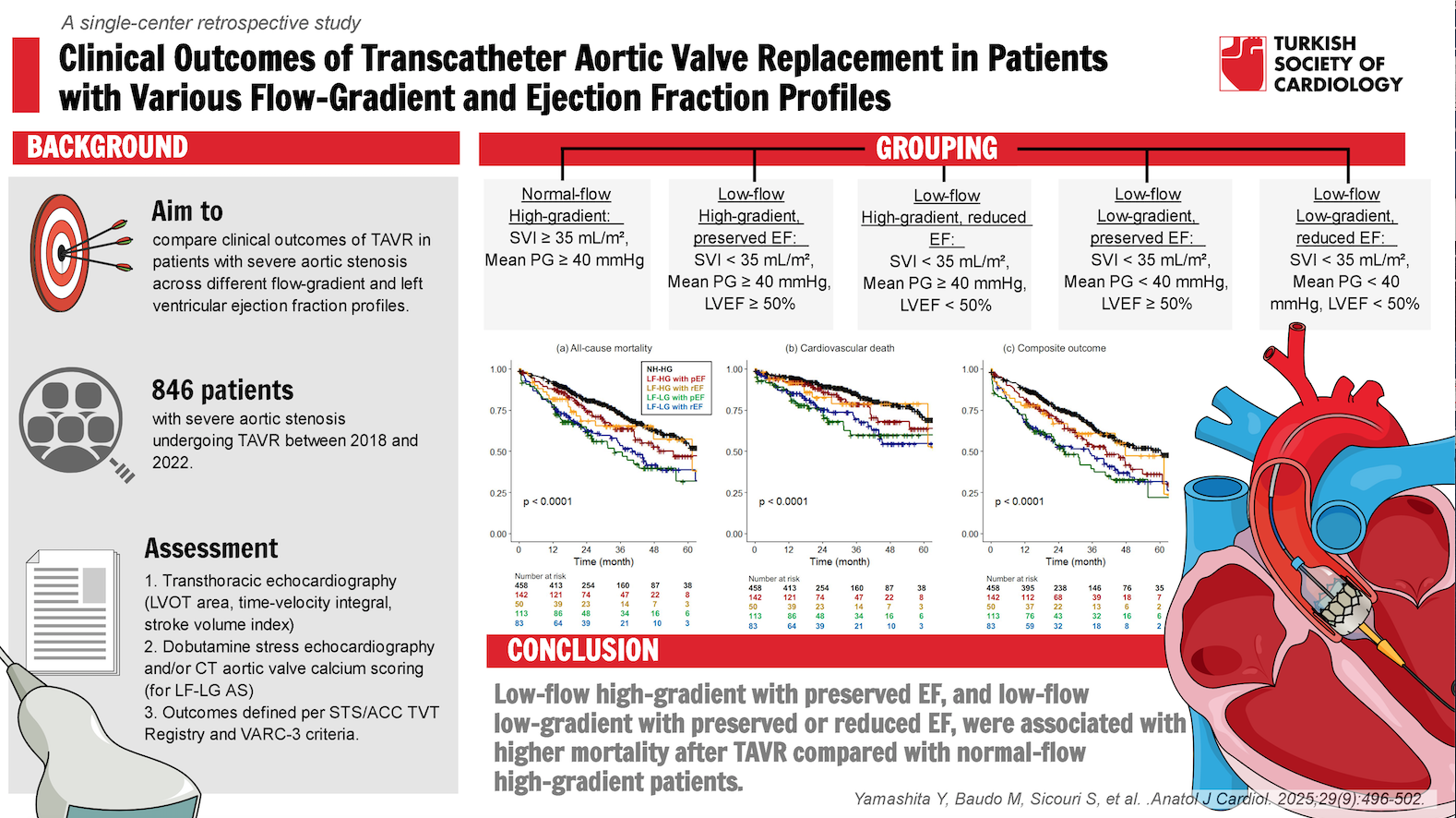
Background: To compare the clinical outcomes of transcatheter aortic valve replace-ment (TAVR) for severe aortic stenosis (AS) in patients with different flow-gradient and left ventricular ejection fraction (EF) profiles.
Methods: Patients with severe AS who underwent TAVR with newer generation valves (Sapien3/3 Ultra, Evolut Pro/Pro+/FX) were retrospectively analyzed. Patients were divided into 5 groups: normal-flow high-gradient (NF-HG) AS (stroke volume index ≥ 35 mL/m2 and mean pressure gradient ≥ 40 mm Hg), low-flow high-gradient (LF-HG) with preserved EF (pEF, ≥ 50%), LF-HG with reduced EF (rEF), low-flow low-gradient (LF-LG) with pEF, and LF-LG with rEF.
Results: A total of 846 patients were included in this study (NF-HG, n = 458; LF-HG with pEF, n = 142; LF-HG with rEF, n = 50; LF-LG with pEF, n = 113; LF-LG with rEF, n = 83). For the entire cohort, the median age was 82 years, and the periprocedural mortality rate was 2.1% with the highest rate in the LF-LG with rEF AS (7.2%). The 1-year and 5-year mortality rates were 13% and 51%, respectively. Multivariable Cox regression analysis showed higher all-cause mortality in the LF-HG with pEF (hazard ratio 1.42 [95% CI: 1.02-1.98]), LF-LG with pEF (1.84 [1.32-2.55]), and LF-LG with rEF (1.78 [1.22-2.61]) groups compared with the NF-HG group. Cardiovascular death rates were significantly higher in the LF-LG groups, but not in the LF-HG groups.
Conclusion: In addition to both LF-LG with pEF and rEF AS, LF-HG with pEF AS had a higher all-cause mortality rate after TAVR compared to NF-HG AS.
Graphical Abstract
Highlights
- This single-center retrospective study evaluates the impact of different flow-gradient profiles and left ventricular ejection fraction (EF) on transcatheter aortic valve replacement (TAVR) outcomes.
- Higher mid-term all-cause mortality was observed in patients with low-flow high-gradient aortic stenosis (AS) and preserved EF, in addition to findings in low-flow low-gradient AS with preserved and reduced EF.
- Detailed stratification of flow-gradient profiles plays a key role in assessing risk and prognosis in patients undergoing TAVR for severe AS.
Introduction
Transcatheter Aortic Valve Replacement (TAVR) is an established therapeutic approach for patients with severe aortic stenosis (AS).1-
Methods
Patients
This single-center retrospective study focused on patients with severe native aortic valve stenosis and rEF who underwent TAVR between January 2018 and December 2022. A total of 1170 TAVR procedures were performed during this time period. Of these, 846 patients who underwent TAVR with newer generation transcatheter heart valves (Sapien 3/3 Ultra [Edwards Lifesciences, Irvine, CA, USA] or Evolut Pro/Pro+/FX [Medtronic Inc., Minneapolis, MN, USA]) for severe AS qualified for inclusion in the study. This study excluded patients who TAVR using older generation valves, or valve-in-valve TAVR for failed bioprostheses. In addition, patients with normal-flow low-gradient AS or unknown flow status, as well as cases involving unsuccessful delivery or conversion to open surgery, were excluded in the analysis.
These patients were divided into 5 groups: NF-HG AS (stroke volume index ≥ 35 mL/m2 and mean pressure gradient ≥ 40 mm Hg), LF-HG with pEF (stroke volume index < 35 mL/m2, mean pressure gradient ≥ 40 mm Hg, and LVEF ≥ 50%), LF-HG with rEF (LVEF < 50%), LF-LG with pEF (stroke volume index < 35 mL/m2, mean pressure gradient < 40 mm Hg, and LVEF ≥ 50%), and LF-LG with rEF. Stroke volume was obtained by transthoracic echocardiography using the LV outflow tract area and the LV outflow tract time-velocity integral. The time-velocity integral was averaged over 3 cardiac cycles for patients in sinus rhythm and 5 cycles for patients in atrial fibrillation. The stroke volume was then indexed to body surface area. Low-flow low-gradient severe AS was comprehensively assessed using dobutamine stress echocardiography and/or computed-tomography aortic valve calcium scoring.10
The primary outcomes of interest were all-cause mortality, cardiovascular death, and composite of all-cause mortality and rehospitalization for heart failure. Other outcomes of interest included periprocedural outcomes. Definitions, terminology, and reported outcomes were consistent with the Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapies Registry and the Valve Academic Research Consortium 3 (VARC-3) criteria.11 The decision for the TAVR procedure was made by a dedicated heart team, primarily based on age and surgical risk according to the STS Predicted Risk of Mortality (STS-PROM), as well as patient anatomy and patient-specific factors such as frailty. The study protocol was approved by the Institutional Review Board (IRB 45CFR164.512). Individual patient consent was waived due to the retrospective nature of the study. This study did not involve the use of artificial intelligence-assisted technologies in the production of this work.
Statistical Analysis
Continuous values are presented as median (interquartile range) unless otherwise noted, and the Kruskal-Wallis test was used to compare between groups for these values. Categorical values are reported as numbers (percentages), and the chi-squared test or Fisher’s exact test was used to compare groups. Kaplan-Meier curves with log-rank
Results
Baseline Patient Characteristics
Of the 846 patients, 388 patients (46%) had low flow status. After further subdivision, there were 142, 50, 113, and 83 patients in the LF-HG with pEF, LF-HG with rEF, LF-LG with pEF, and LF-LG with rEF groups, respectively. The median age for the entire cohort was 82 years. Baseline and procedural characteristics are shown in
Periprocedural Outcomes
The overall periprocedural mortality rate was 2.1% with the highest rate in the LF-LG with rEF AS (7.2%,
Mid-Term Outcomes
The median follow-up period was 24 (range 0-72) months, and the overall 1- and 5-year all-cause mortality rates were 13% and 51%, respectively.
Discussion
In this study, outcomes after TAVR in patients were compared with severe AS according to flow-gradient and LVEF status, and observed the following major findings: 1) 46% of the patients had low-flow status determined as a stroke volume index of < 35 mL/m2, 2) both LF-LG with pEF and rEF AS had similarly worse clinical outcomes compared to NF-HG AS (adjusted HRs of 1.98 [95% CI 1.47-2.65] and 2.21 [1.59-3.07] for all-cause mortality, respectively), 3) In addition, LF-HG with pEF was associated with higher all-cause mortality and a composite of all-cause mortality and rehospitalization for heart failure compared to NF-HG AS. The overall periprocedural, 1-year, and 5-year mortality rates (2.1%, 13%, and 51%, respectively) in this study are consistent with recent reports on TAVR outcomes.12,
To date, considerable attention has been paid to the outcomes of TAVR in patients with LF-LG AS, and LF-LG with rEF appears to be associated with worse outcomes compared to high-gradient AS.6-
To date, studies focusing on the effects of LF-HG AS are limited. However, Maréchaux et al9 showed that LF-HG status displayed considerable mortality risk during follow-up compared with NF-HG status in patients with asymptomatic or minimally severe AS with pEF. In addition, Mangner et al14 observed higher all-cause mortality in patients with LF-HG AS compared to NF-HG AS at 3 years after TAVR (38% versus 25%). The results, which focus solely on newer-generation valves, are likely to reinforce these earlier findings and more closely reflect current practice. In the Simvastatin Ezetimibe in Aortic Stenosis study,17 they suggested that patients with low-flow AS exhibit a higher global LV load and a higher prevalence of myocardial systolic dysfunction, which cannot be predicted by assessing LVEF alone. The combination of increased global LV afterload and decreased cardiac output in the low-flow group suggests diminished cardiac reserve. Chronic exposure to elevated afterload eventually exceeds the limit of compensatory capacity of the LV, leading to intrinsic impairment of myocardial function and reduced cardiac output.5 This pathophysiologic mechanism may help explain the worse prognosis observed in low-flow patients in the present study. This study highlights the essential role of detailed flow-gradient status stratification in risk and prognosis assessment for TAVR in patients with severe AS, including the introduction of the “LF-HG AS” concept. Adopting this approach could lead to the development of more tailored treatment strategies. On the other hand, this study found no significant prognostic differences between LF-HG with rEF and NF-HG AS. It is unclear whether this suggests that reduced stroke volume due to a smaller LV cavity, rather than due to rEF, has a more detrimental effect on TAVR outcomes, or whether this observation is simply due to lack of statistical power resulting from the small sample size of patients with LF-HG AS and rEF. In addition, conflicting results were suggested by Alkhalil et al,8 who reported that low flow was an independent predictor of adverse events in the rEF subgroup, but not the pEF subgroup, in patients with high-gradient AS. Further research with larger cohorts is needed to accurately assess the true impact in this specific patient population.
According to current American College of Cardiology/American Heart Association guidelines, aortic valve intervention is recommended only for symptomatic patients with AS and pEF or LF-LG AS, and for asymptomatic patients with severe AS and pEF under certain conditions such as very severe AS and low surgical risk.4 It is critical to consider the timing of the intervention in terms of both procedural risks and long-term benefits. In this study, a higher periprocedural mortality was found in the LF-LG with rEF group, whereas similar periprocedural mortality were observed in the LF-HG and LF-LG with pEF AS compared to the NF-HG AS. Given the potentially worse follow-up mortality in the LF-HG with pEF and LF-LG AS, the optimal timing of the intervention should also be reconsidered. It has also been reported that patients with LF-HG AS are less likely to undergo aortic valve intervention than patients with high-gradient AS,18 and these patients typically have more delayed referral for interventional evaluation, which may contribute to worse outcomes.10 In addition, Ludwig et al19 reported that among patients with non-severe LF-LG AS and rEF, TAVR represents a major predictor of superior survival compared with those on medical management. Further research is needed to investigate whether earlier detection, referral, and intervention for these conditions could lead to beneficial outcomes.
Study Limitations
This study is constrained by several notable limitations. Firstly, being a single-center retrospective study with a relatively small patient cohort, there are concerns regarding the statistical power and overall robustness of the findings. It may also limit the generalizability of the findings to other institutions with different patient populations or procedural strategies. Although several clinical and procedural variables were adjusted for, unmeasured confounders such as frailty, the severity of prior heart failure, or other comorbidities, were not captured in this dataset and may have influenced the outcomes. In addition, there are recognized technical difficulties in performing accurate echocardiographic assessments, for example, in measuring the calcified LV outflow tract in cases of severe AS,20 which may lead to misclassification of flow status. Another limitation is the absence of data on the preoperative waiting period and follow-up reverse flow data from echocardiography, which restricts the ability to perform a more comprehensive analysis incorporating these aspects. Furthermore, the choice of transcatheter heart valve type (balloon-expandable vs. self-expanding) was at the discretion of the interventionist, which may have introduced a treatment selection bias. Although valve type in the multivariable analysis was adjusted for and no significant impact on the endpoints was found, unmeasured confounding related to device selection cannot be completely excluded. Despite these challenges, this study is still clinically significant as it highlights the utility of non-invasive and commonly assessed parameters in predicting mid-term outcomes.
Conclusion
This single-center retrospective study showed that, in addition to both LF-LG with pEF and rEF AS, LF-HG with pEF AS had a higher all-cause mortality rate after TAVR compared to NF-HG AS.
Supplementary Materials
Footnotes
References
- Mack MJ, Leon MB, Thourani VH. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389(21):1949-1960.
- Reardon MJ, Van Mieghem NM, Popma JJ. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331.
- Vahanian A, Beyersdorf F, Praz F. ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2021;17(14):e1126-e1196.
- Otto CM, Nishimura RA, Bonow RO. ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2020;162(2):e183-e353.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115(22):2856-2864.
- Muratori M, Fusini L, Tamborini G. Outcomes of transcatheter aortic valve replacement patients with different transvalvular flow-gradient patterns. Am J Cardiol. 2023;209():173-180.
- Wagener M, Reuthebuch O, Heg D. Clinical outcomes in high-gradient, classical low-flow, low-gradient, and paradoxical low-flow, low-gradient aortic stenosis after transcatheter aortic valve implantation: A report from the SwissTAVI registry. J Am Heart Assoc. 2023;12(12):e029489-.
- Alkhalil M, Brennan P, McQuillan C. Flow, reflected by stroke volume index, is a risk marker in high-gradient aortic stenosis patients undergoing transcatheter aortic valve replacement. Can J Cardiol. 2020;36(1):112-118.
- Maréchaux S, Rusinaru D, Altes A, Pasquet A, Vanoverschelde JL, Tribouilloy C. Prognostic value of low flow in patients with high transvalvular gradient severe aortic stenosis and preserved left ventricular ejection fraction: A multicenter study. Circ Cardiovasc Imaging. 2019;12(10):e009299-.
- Sharma N, Sachedina AK, Kumar S. Low-flow, low-gradient severe aortic stenosis: a review. Heart Int. 2023;17(1):8-12.
- Généreux P, Piazza N. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77(21):2717-2746.
- Arnold SV, Manandhar P, Vemulapalli S. Mediators of improvement in TAVR outcomes over time: insights from the STS-ACC TVT registry. Circ Cardiovasc Interv. 2023;16(7):e013080-.
- Hahn RT, Webb J, Pibarot P. 5-year follow-up from the PARTNER 2 aortic valve-in-valve registry for degenerated aortic surgical bioprostheses. JACC Cardiovasc Interv. 2022;15(7):698-708.
- Mangner N, Stachel G, Woitek F. Predictors of mortality and symptomatic outcome of patients with low-flow severe aortic stenosis undergoing transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7(8):e007977-.
- Osman M, Ghaffar YA, Foster T. Meta-analysis of outcomes of transcatheter aortic valve implantation among patients with low gradient severe aortic stenosis. Am J Cardiol. 2019;124(3):423-429.
- Ribeiro HB, Lerakis S, Gilard M. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: the TOPAS-TAVI registry. J Am Coll Cardiol. 2018;71(12):1297-1308.
- Cramariuc D, Cioffi G, Rieck AE. Low-flow aortic stenosis in asymptomatic patients valvular–arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging. 2009;2(4):390-399.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31(3):281-289.
- Ludwig S, Schofer N, Abdel-Wahab M. Transcatheter aortic valve replacement in patients with reduced ejection fraction and nonsevere aortic stenosis. Circ Cardiovasc Interv. 2023;16(5):e012768-.
- Baumgartner HC, Hung JC-C, Bermejo J. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18(3):254-275.


