2Department of Cardiovascular Surgery, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, China
3Department of Radiology, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, China
Abstract
Background: The presence of constrictive pericarditis (CP) in conjunction with tricuspid regurgitation (TR) and the worsening of TR following pericardiectomy are associated with a reduction in patient survival. The purpose of this study was to investigate the prevalence of tuberculous CP in conjunction with TR, the incidence of worsening regurgitation following pericardiectomy, and the analysis of associated factors.
Methods: Seventy-five consecutive patients who underwent pericardiectomy for tuberculous CP at the institution between January 2021 and December 2023 were retrospectively analyzed. Their clinical, imaging, and hemodynamic characteristics were analyzed.
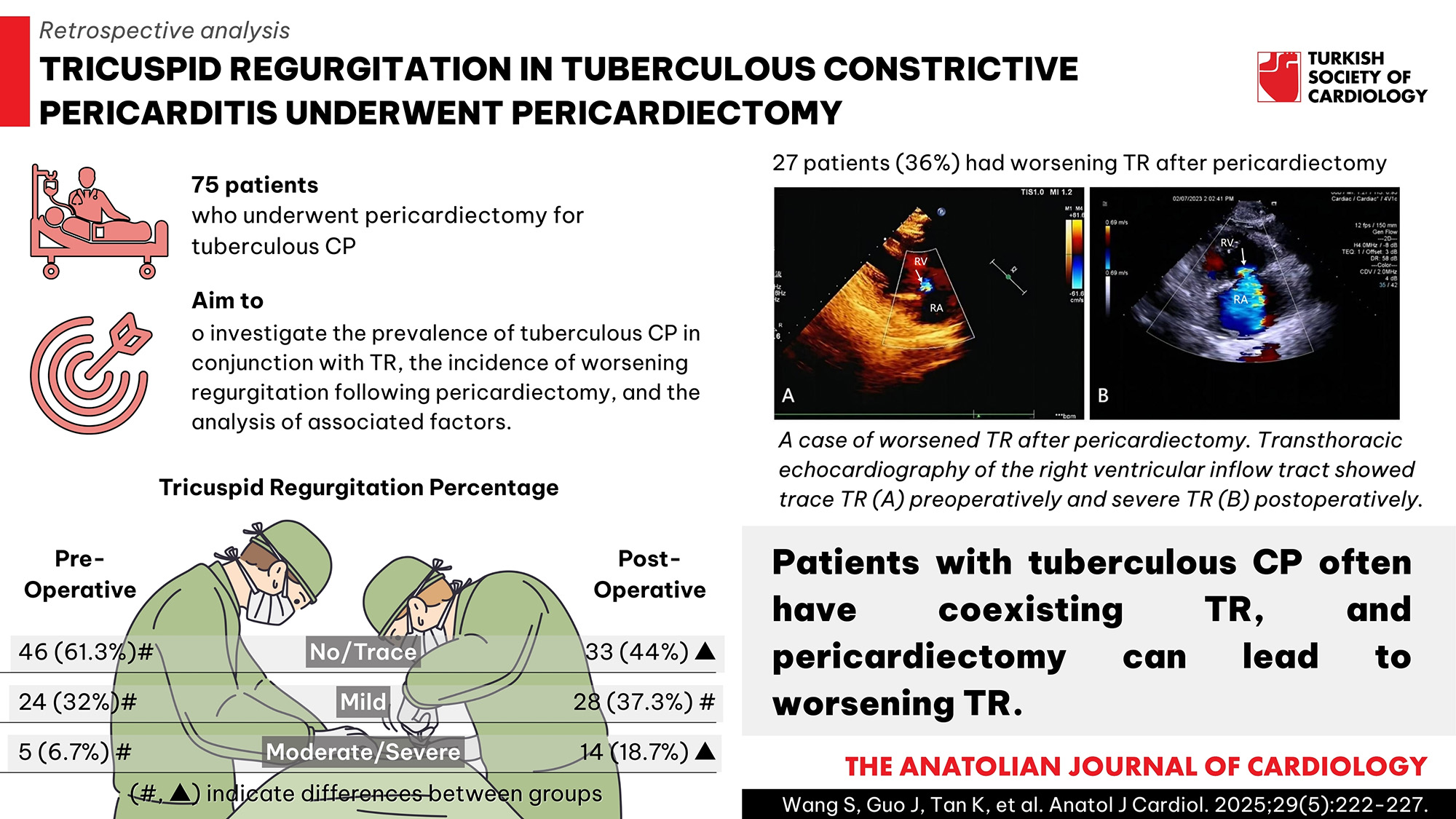
Results: Among the 75 patients with tuberculous CP, 29 patients (38.7%) had mild or greater TR preoperatively and 27 patients (36%) had worsening TR after pericardiectomy. In patients with worsening TR, the pericardial thickness of the right ventricular (RV) lateral wall was significantly thickened preoperatively, and there was a reduction in the tricuspid annular plane systolic excursion (TAPSE), right ventricle S’ tissue Doppler velocity (S’), and right ventricle fractional area change (FAC) postoperatively. The preoperative inferior vena cava diameter and the postoperative right atrial and RV basal diameters were significantly larger in patients with worsening TR compared with patients with non-worsening TR, whereas the TAPSE, S’, and FAC were significantly lower before and after the surgery (P < .05). The FAC [OR = 0.354; 95% CI (0.165-0.761), P = .008] and pericardial thickness of the RV lateral wall [OR = 1.887; 95% CI (1.206-2.953), P = .005] were independently associated with worsening TR.
Conclusion: Patients with tuberculous CP often have coexisting TR, and pericardiectomy can lead to worsening TR. The pericardial thickness of the RV lateral wall and FAC are independently associated with worsening TR following pericardiectomy.
Graphical Abstract
Highlights
- This research reveals that tuberculous constrictive pericarditis coexisted with mild or greater tricuspid regurgitation (TR), accounting for 38.7% preoperatively.
- Pericardiectomy can lead to worsening TR, with an incidence of 36%.
- The research highlights the independent factors associated with the worsening of TR following surgery, including the right ventricle fractional area change and the pericardial thickness of the right ventricular lateral wall.
Introduction
Constrictive pericarditis (CP) results in impaired ventricular diastolic filling due to thickening and fibrosis of the pericardium, which ultimately leads to right heart failure. Tuberculosis infection is the main cause of CP in developing countries, accounting for approximately 38%-83% of all CP, and currently, pericardiectomy is the only effective treatment for CP.1,
Methods
Study Population
The study was a retrospective single-center observational study and enrolled a total of 86 patients with tuberculous CP who underwent successful pericardiectomy from January 2021 to December 2023 in our hospital. All patients had a history of anti-tuberculosis treatment before surgery or pathologically confirmed tuberculous pericarditis postoperatively, with pre- and postoperative echocardiography available. Eleven patients were excluded from analysis as they underwent other concomitant cardiac operations at the time of pericardiectomy, of whom 5 underwent coronary artery bypass grafting, 3 underwent valvuloplasty, 1 underwent valve replacement, and 2 underwent a secondary pericardiectomy. Seventy-five patients were finally included. Clinical data including demographic information, past history, and laboratory tests were collected. The study was conducted in accordance with the Declaration of Helsinki and was approved by our institutional ethics committee. Because this was a retrospective study, written informed consent for this study was waived.
Surgical Technique
Pericardiectomy was performed via median sternotomy in all cases, without cardiopulmonary bypass. The primary surgical goal was total pericardiectomy, which was defined as the excision of the anterior pericardium up to the phrenic nerves and the diaphragmatic surface. The visceral and parietal pericardium were removed whenever technically possible. Any excision less than total was defined as partial.
Assessment of Cardiac Imaging
Preoperative computed tomography (CT) was performed to measure the pericardial thickness of the right ventricular (RV) lateral wall, as well as to observe whether the thickened pericardium was combined with calcification or pericardial effusion. Pericardial thickness >3 mm was considered to be pericardial thickening.6
All patients underwent transthoracic echocardiography 1-3 days preoperatively and 7-10 days postoperatively, using the Philips EPIQ 7C (Philips Medical Systems, Andover) or ACUSON SC2000 (Siemens Medical Solutions USA, Mountain View, CA) equipment. Valve regurgitation grading was based on multiparametric criteria of echocardiography guidelines, taking into account vena contracta width (VCW) and color flow jet area (quantified), along with visual assessment of tricuspid valve morphology, color, and continuous wave Doppler properties.7 Tricuspid regurgitation was classified as no regurgitation, trace regurgitation (regurgitant bundle confined to the orifice and VCW <2 mm), mild regurgitation (2 mm ≤ VCW <3 mm and jet area <5 cm2), moderate regurgitation (3 ≤ VCW <7 mm and 5 ≤ jet area <10 cm2), and severe regurgitation (VCW ≥7 mm and jet area ≥10 cm2), where no regurgitation and trace regurgitation were considered to be at the same grade. Worsening TR was defined as a postoperative increase in regurgitation of at least one grade compared with preoperative TR. Routine parameters of cardiac structure and function were recorded, of which indicators of RV systolic function included right ventricle fractional area change (FAC), the tricuspid annular plane systolic excursion (TAPSE), and right ventricle S’ tissue Doppler velocity (S’).
Statistical Analysis
Statistical analysis was performed using SPSS Statistics version 26.0 (SPSS, Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to test for normality. Normally distributed data are presented as mean ± SD, and non-normally distributed data are presented as median [M (Q25, Q75)]. Continuous numeric data were compared using the independent samples
Results
Evolution of Tricuspid Regurgitation
Seventy-five patients who underwent pericardiectomy for tuberculous CP in the study were retrospectively analyzed. In the majority of patients (61.3%), preoperative TR was none or trace, with only 6.7% exhibiting moderate regurgitation. The incidence of postoperative moderate/severe TR was found to be significantly higher in 18.7% of patients, as shown in
Comparison of General Clinical Data
Patients with worsening TR had higher C-reactive protein (CRP) and longer duration of the disease compared to patients with non-worsening TR (
Comparison of Computed Tomography Parameters
Patients with worsening TR had thicker pericardial thickness in the RV lateral wall and more patients with pericardial thickening and calcification compared to those without worsening TR (
Comparison of Echocardiographic Parameters
In patients with worsening TR, RV basal diameter, VCW, and colored flow jet area of TR were significantly increased, whereas TAPSE, S’, and FAC were decreased after pericardiectomy (
Preoperative inferior vena cava diameters were increased in patients with worsening TR compared with those without worsening TR. In addition, right atrial and RV basal diameters were significantly increased postoperatively. In contrast, S’, TAPSE, and FAC decreased significantly both before and after surgery (
Multiple logistic regression analysis showed that FAC [OR = 0.354; 95% CI (0.165-0.761),
Discussion
The results of the present study show the following:① the coexistence of mild or greater TR and tuberculous CP is present in 38.7% of patients preoperatively. ② Pericardiectomy can lead to worsening TR in 36% of patients.③ Patients with worsening TR have enlarged right ventricles and reduced RV function postoperatively. ④ The pericardial thickness of the RV lateral wall and FAC are independently associated with worsening TR following pericardiectomy.
Tricuspid regurgitation can result from primary, secondary, or multiple causes and is strongly associated with high morbidity and mortality and was previously an underestimated clinical problem.3 Tricuspid regurgitation is a common and important comorbidity of CP, and studies have shown that moderate/severe TR is an independent predictor of late postoperative mortality in CP (OR = 2.9, 95% CI: 1.5-5.6).8 However, previous studies on the prevalence of TR coexisted with CP were scarce and highly variable. In this study, the prevalence of preoperative TR coexisted with tuberculous CP was shown to be 38.7% (of which 32% were mild and only 6.7% were moderate), and 3 patients who underwent valve repair for severe regurgitation were excluded from this study. This is similar to the results of Calderon–Rojas, which included 518 patients and showed mild TR in 37% (191 patients) and moderate/severe in 10% (51 patients), but the study included mainly cardiac surgeries related to CP and did not include tuberculous CP.4 A study by Góngora et al8 on combined TR in 261 patients who underwent pericardiectomy showed that moderate/severe TR accounted for 20% of the cases (54 patients), but there were 9 pacemaker implantations (3%) and 15 cases of radiation pericarditis (6%) among these 54 patients. The prevalence of coexisting TR in CP of different etiologies varies, which may be strongly related to the comorbid underlying disease of the study population, such as a history of pacemaker implantation, atrial fibrillation, pulmonary disease, etc. There is noconsensus on the mechanism of coexisting TR in CP, preferring to believe that TR is secondary to the structural and functional remodeling of the patient’s right atrium and right ventricle during the pathological processes of CP.5
Cardiac surgery can lead to worsening TR, with an incidence of approximately 17%-50%, and is associated with a reduction in long-term patient survival after surgery.9,
This study showed that FAC was an independent factor contributing to postoperative worsening TR, suggesting that the overall contractile function of the RV is more closely related to the worsening of TR. Although the RV myocardium is predominantly subepicardial longitudinal myocardium, which plays a major role in contractile function, the degree of damage to the RV myocardium of the corresponding segments is different due to the different sites and degrees of pericardial thickening in patients with CP. Tricuspid annular plane systolic excursion and S’ can only reflect the longitudinal contractile function in the localized area (proximal to the annulus) rather than the overall contractile function.17 Therefore, in tuberculous CP, the overall contractile function of the RV should be emphasized before surgery to prevent worsening of TR.
In this study, it was also found that the RV lateral wall pericardium was thicker and more often combined with calcification in patients with worsening TR, and the thickness of the RV lateral wall pericardium was an influencing factor in the worsening of TR. The pericardial thickness was negatively correlated with the longitudinal strain of the RV wall in the study by Kusunose et al.18 The degree of compression of the cardiac chambers is directly proportional to the thickness of the pericardium. Additionally, the adherence of the pericardium to the RV wall is enhanced in cases of thickening or calcification, resulting in greater local epicardial myocardial damage and increased difficulty in separating it during surgery. This, in turn, contributes to a further reduction in RV systolic function.
This study found that preoperative CRP was higher in patients with worsening TR. It is a sensitive indicator for assessing the state of infection and inflammation and is associated with the prognosis of the disease. Fernandes et al showed an association between high CRP levels and death in patients after pericardiectomy.19 This detail should not be overlooked when evaluating patients preoperatively.
Study Limitations
It should be noted that this study is subject to a number of limitations. Firstly, this was a single-center surgical experience, which may have introduced a degree of selection bias. Secondly, the tricuspid annulus was not subjected to analysis in order to ascertain the role of annular dilatation in the worsening of TR. Thirdly, the perioperative data collected in this study lack clarity regarding the mid- and long-term follow-up and survival outcomes of patients with worsened TR after tuberculous CP, which represents a gap in the literature that requires further investigation.
Conclusion
Tricuspid regurgitation often complicates patients with tuberculous CP, and pericardiectomy can lead to worsening TR. The thickness of the pericardium of the RV lateral wall and FAC are independently associated with worsening TR following pericardiectomy. It is imperative that clinicians monitor TR in patients with tuberculous CP, both pre- and post-operatively, in order to facilitate the development and adjustment of treatment programs.
It is confirmed that artificial intelligence (AI)–assisted technologies (such as Large Language Models [LLMs], chatbots, or image creators) were not used in the production of the submitted work.
Footnotes
References
- Cinar B, Enç Y, Göksel O. Chronic constrictive tuberculous pericarditis: risk factors and outcome of pericardiectomy. Int J Tuberc Lung Dis. 2006;10(6):701-706.
- Liu VC, Fritz AV, Burtoft MA, Martin AK, Greason KL, Ramakrishna H. Pericardiectomy for constrictive pericarditis:analysis of outcomes. J Cardiothorac Vasc Anesth. 2021;35(12):3797-3805.
- Dahou A, Levin D, Reisman M, Hahn RT. Anatomy and physiology of the tricuspid valve. JACC Cardiovasc Imaging. 2019;12(3):458-468.
- Calderon-Rojas R, Greason KL, King KS. Tricuspid valve regurgitation in patients undergoing pericardiectomy for constrictive pericarditis. Semin Thorac Cardiovasc Surg. 2020;32(4):721-728.
- Tabucanon RS, Wang TKM, Chetrit M. Worsened tricuspid regurgitation following pericardiectomy for constrictive pericarditis. Circ Cardiovasc Imaging. 2021;14(10):e012948-.
- Senapati A, Isma’eel HA, Kumar A. Disparity in spatial distribution of pericardial calciffcations in constrictive pericarditis. Open Heart. 2018;5(2):e000835-.
- Zoghbi WA, Adams D, Bonow RO. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30(4):303-371.
- Góngora E, Dearani JA, Orszulak TA, Schaff HV, Li Z, Sundt TM. Tricuspid regurgitation in patients undergoing pericardiectomy for constrictive pericarditis. Ann Thorac Surg. 2008;85(1):163-170.
- Matsunaga A, Duran CMG. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005;112(9 suppl):I453-I457.
- Cremer PC, Wang TKM, Rodriguez LL. Incidence and clinical significance of worsening tricuspid regurgitation following surgical or transcatheter aortic valve replacement: analysis from the PARTNER IIA trial. Circ Cardiovasc Interv. 2021;14(8):e010437-.
- Johnson TL, Bauman WB, Josephson RA. Worsening tricuspid regurgitation following pericardiectomy for constrictive pericarditis. Chest. 1993;104(1):79-81.
- Yu HT, Ha JW, Lee S. Transient right ventricular dysfunction after pericardiectomy in patients with constrictive pericarditis. Korean Circ J. 2011;41(5):283-286.
- Choudhry MW, Homsi M, Mastouri R, Feigenbaum H, Sawada SG. Prevalence and prognostic value of right ventricular systolic dysfunction in patients with constrictive pericarditis who underwent pericardiectomy. Am J Cardiol. 2015;116(3):469-473.
- Akasaka T, Yoshida K, Yamamuro A. Phasic coronary flow characteristics in patients with constrictive pericarditis: comparison with restrictive cardiomyopathy. Circulation. 1997;96(6):1874-1881.
- Levine HD. Myocardial fibrosis in constrictive pericarditis. Electrocardiographic and pathologic observations. Circulation. 1973;48(6):1268-1281.
- Homsi M, Mahenthiran J, Vaz D, Sawada SG. Reduced right ventricular systolic function in constrictive pericarditis indicates myocardial involvement and persistent right ventricular dysfunction and symptoms after pericardiectomy. J Am Soc Echocardiogr. 2007;20(12):1417.e1417-.e14177.
- Dell’Italia LJ. Anatomy and physiology of the right ventricle. Cardiol Clin. 2012;30(2):167-187.
- Kusunose K, Dahiya A, Popović ZB. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging. 2013;6(3):399-406.
- Fernandes F, Melo DTPD, Ramires FJA. Importance of clinical and laboratory findings in the diagnosis and surgical prognosis of patients with constrictive pericarditis. Arq Bras Cardiol. 2017;109(5):457-465.


