2Department of Cardiology, Kocaeli City Hospital, Kocaeli, Türkiye
3Department of Cardiology, Kartal Koşuyolu Training and Research Hospital, İstanbul, Türkiye
4Department of Cardiology, Faculty of Medicine, Medipol University, İstanbul, Türkiye
5Center for Coronary Artery Disease, Minneapolis Heart Institute and Minneapolis Heart Institute Foundation, Minneapolis, Minnesota, USA
6Department of Cardiology, Hisar Intercontinental Hospital, Nişantaşı University, İstanbul, Türkiye
Abstract
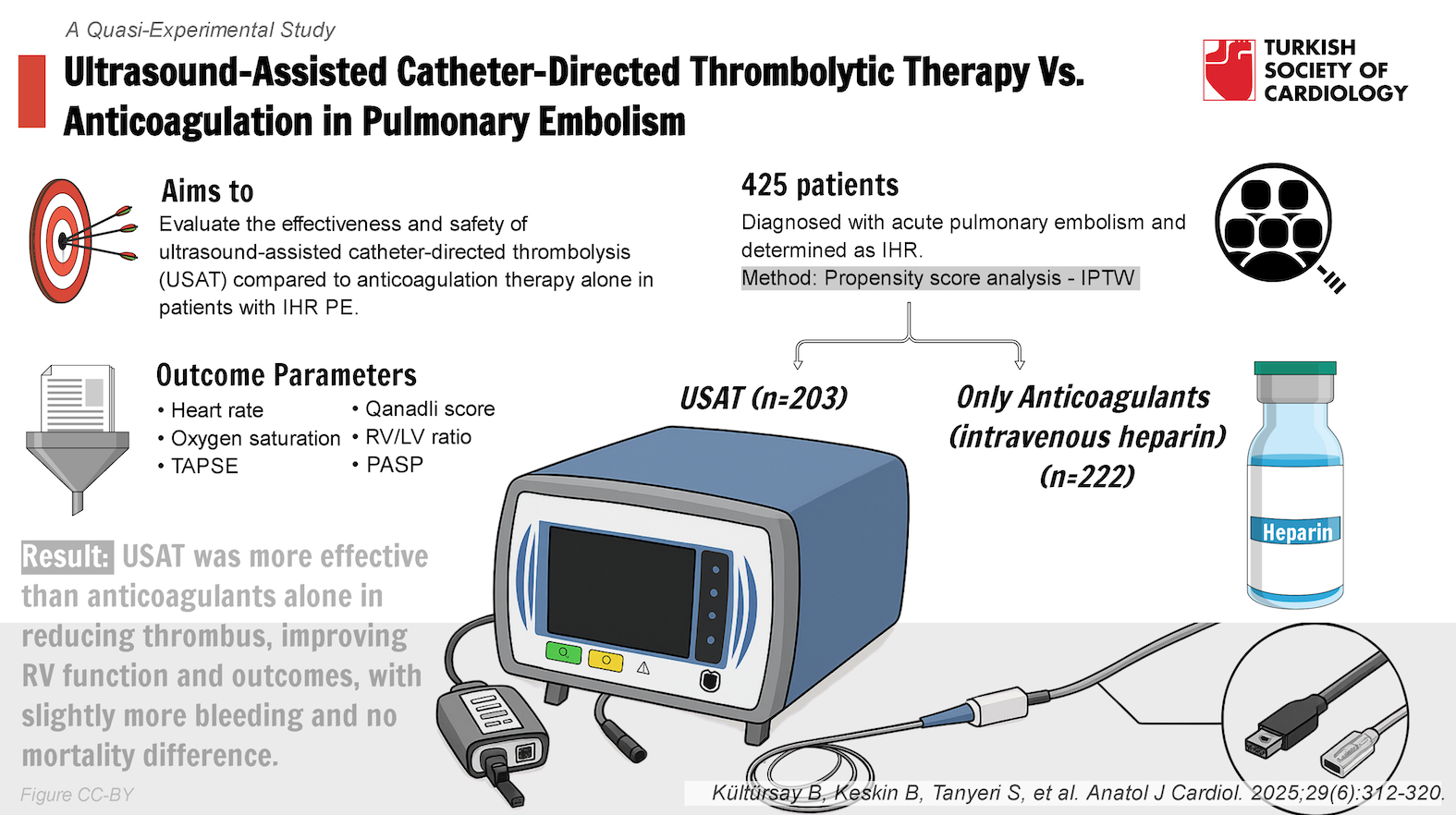
Background: Given the bleeding risk associated with full-dose intravenous thrombolytic treatment and the absence of randomized clinical trial evidence, current guidelines do not recommend reperfusion treatments as first-line therapy for intermediate-high risk (IHR) pulmonary embolism (PE). The aim of this study was to evaluate the effectiveness and safety of ultrasound-assisted catheter-directed thrombolysis (USAT) compared to anticoagulation therapy alone in patients with IHR PE.
Methods: A total of 425 patients diagnosed with acute PE and determined as IHR, 203 of whom underwent USAT, and 222 patients receiving only anticoagulants as the control group, were included. Baseline and post-treatment right ventricle (RV) function in echocardiography, tomographic RV/left ventricle (RV/LV) ratio, Qanadli score (Qs), and % changes from baseline were taken as primary effectiveness outcomes. For safety outcomes, major and minor bleeding and in-hospital all-cause death were adopted. Propensity score analysis was performed to reduce confounders and bias.
Results: The USAT treatment was found to be associated with improved RV function and decreased Qs, but no significant effect was observed on the RV/LV ratio and its change. Bleeding events were more frequent in the USAT group (P < .001 for both), and no difference was observed in terms of mortality.
Conclusion: The study, based on real-life data, has shown that a moderate-dose, slow-infusion tissue-type plasminogen activator regimen is superior to anticoagulant therapy alone in terms of reducing pulmonary arterial thrombus burden, restoring RV dysfunction, and improving clinical outcomes in acute PE patients at IHR. However, it has also resulted in a slight increase in bleeding events.
Graphical Abstract
Highlights
- This study investigates the effectiveness and safety of ultrasound-assisted catheter-directed thrombolysis (USAT) vs. anticoagulation alone in patients with intermediate-risk pulmonary embolism (PE), given the limited options for reperfusion therapies.
- A total of 425 patients diagnosed with intermediate-risk acute PE were included, with 203 receiving USAT and 222 receiving standard anticoagulant therapy. Propensity score analysis was performed to allow robust comparisons.
- The USAT group demonstrated significant improvements in right ventricular function, Qanadli score, heart rate, oxygen saturation, and pulmonary artery pressure compared to the anticoagulant-only group.
- While USAT showed benefits in thrombus reduction and right ventricle function, it was associated with higher rates of bleeding, including major (5.9% vs. 0.9%) and minor (9.4% vs. 1.4%) events compared to anticoagulant therapy.
- The findings suggest that while USAT offers superior outcomes in managing intermediate-risk PE, clinicians must weigh these benefits against the increased risk of bleeding when considering treatment options.
Introduction
Despite recent advancements in prevention, diagnosis, and anticoagulant treatment, acute pulmonary embolism (PE) continues to be a major cause of global morbidity and mortality.1,
Methods
Study Design and Population
A total of 425 patients out of 946 patients diagnosed with acute PE in the tertiary cardiovascular center between October 2012 and April 2023 were included, with 203 of them undergoing USAT and 222 receiving only anticoagulants as the control group. The systematic work-up for the initial diagnosis of acute PE and risk stratification, including multidetector contrast-enhanced computed tomography (CT) angiography, echocardiography assessments, PE severity indexes (PESI), and biomarker evaluation, was based on the criteria recommended by the ESC/ERS 2014 and 2019 PE guidelines, and all patients were at IHR.1,
Chest Computed Tomography Pulmonary Angiography and Echocardiography
Computed tomography images were acquired using 64-slice helical CT angiography (Toshiba Aquilion 64™, Toshiba Medical Systems Corp., Tokyo, Japan). A validated CT score for pulmonary arterial (PA) occlusion proposed by Qanadli et al.6 [Qanadli score (QS)], right ventricle (RV) to left ventricle (LV) ratio, RV diameter, right atrial to left atrial diameter ratio (RA/LA ratio), and main, left, and right PA diameters were measured from CT images. Pulmonary infarction is defined as a peripheral wedge-shaped pulmonary consolidation in a hypoperfused segment of the lung. The CT images were evaluated at admission and 72-96 hours after the initiation of treatment. Transthoracic echocardiography (TTE) was performed on all patients on the first day of admission and repeated at discharge. Tricuspid annular plane systolic excursion (TAPSE) and tissue Doppler (S’) measurements were obtained to assess RV function in TTE, and estimated pulmonary artery pressures (PAPs) were calculated from the tricuspid regurgitation jet. All measurements and assessments were made in accordance with the American Society of Echocardiography guidelines.7
Righ Heart Catheterization, Pulmonary Angiography, and Ultrasound-Assisted Catheter-Directed Thrombolysis Procedure
Only the femoral venous route with a 6-French (F) sheath was used, and arterial puncture was avoided. A 6F multipurpose catheter was used for initial PA pressure measurements and selective angiograms. The EkoSonic Endovascular Device (EKOS, Bothell, Washington) was employed, which includes the Intelligent Drug Delivery Catheter (IDDC) and the MicroSonic Device (MSD) equipped with multiple small ultrasound transducers distributed across the treatment zone. A 0.035-inch hydrophilic guidewire was used to navigate through the thrombotic segment of the target pulmonary artery (PA). Once positioned safely within a large segmental PA, the multipurpose catheter was exchanged for the IDDC of the USAT system. After removing the guidewire, the MSD was inserted and advanced through the IDDC, then connected to the EkoSonic control unit. Recombinant tissue-type plasminogen activator (tPA) was used as the thrombolytic agent, with a continuous infusion of tPA based on the selected dose and duration, and saline coolant at 35 mL/h per catheter was initiated. The preferred treatment approach involved operator-driven selection of tPA dose and treatment duration on an individual basis, tailored to each patient’s risk status and comorbidities. Approximately 4 hours after the completion of tPA delivery, the system catheters and sheath were removed under fluoroscopic control. Intravenous heparin was started after termination of the USAT procedure, and the aim was to keep the aPTT around 60 seconds.
Primary Measures of Effectiveness
As measures of treatment effectiveness, TAPSE and its change were assessed using TTE, while baseline and post-treatment RV/LV ratio, PA obstruction severity (Qs), and their changes from baseline were evaluated using CT.
Safety Measures
For safety endpoints, major and minor bleeding events, as well as in-hospital deaths from all causes, were recorded. Major bleeding was defined as overt hemorrhage associated with a fall in the hemoglobin level ≥2.0 g/dL or with transfusion of 2 units of packed red blood cells, or involvement of critical site bleeding including airway, intra-abdominal, intracranial, and other central nervous system bleeding, pericardial tamponade, hemothorax, and retroperitoneal hematoma. Clinically overt bleeding not fulfilling the criteria of major bleeding was classified as a minor bleeding complication.8
Statistical Analysis and Modelling
Normally distributed continuous data were expressed as mean and standard deviation values, whereas non-normally distributed data were expressed as medians and interquartile ranges, and categorical data were described as absolute and percentage values. Independent samples
All baseline models included PESI and the RV/LV1 ratio, while other variables were added based on previous studies, clinical experience, expert opinions, and variables found to be significant (
For all statistical analyses, 2-tailed probability (
Results
The baseline characteristics and clinical data of patients who underwent USAT and those who received only anticoagulants are presented in
When admission RV function was assessed by TTE using TAPSE and S’, no difference was found between the 2 groups. The estimated echocardiographic PAPs were higher in the USAT group (
Catheter pressures, tPA duration and doses, and procedural details are presented in
Post-treatment clinical, echocardiographic, and tomographic measurements of the patients are summarized in
When examining the effect of variables on RV/LV change as an outcome, it was found that a higher baseline RV/LV1 ratio was associated with an increase in change, while the PESI score was associated with a decrease in change (
As for the effect of variables on Qs change, it was found that a high pre-treatment Qs1 and the application of USAT increased Qs change, while a history of heart failure reduced Qs change. For Qs2, it was determined that a high Qs1 was associated with an increase in Qs2, while USAT application and high TAPSE at baseline were associated with low Qs2.
It was found that the PESI score and TAPSE2 negatively affected TAPSE change, while the application of USAT increased TAPSE change. When examining the effect of variables on TAPSE2, a history of atrial fibrillation was associated with low TAPSE2, whereas USAT application and high TAPSE1 were associated with high TAPSE2.
From a safety perspective, it was found that the USAT group had a higher incidence of major and minor bleeding compared to the anticoagulant group, but no difference was observed in mortality due to any cause during hospitalization (Supplementary Tables).
Discussion
The study represents the largest single-center data comparing USAT and anticoagulant treatment in patients with acute PE at IHR. In this study, it was demonstrated that USAT is superior to anticoagulant treatment in terms of effectiveness, showing greater reduction in thrombus burden, improvement in RV function, and better clinical parameter outcomes. In the USAT group, the significant reduction in PAP, confirmed by invasive measurements, also supports this result. Although the superiority of USAT in terms of effectiveness has been demonstrated, this increased effectiveness is also associated with a slightly elevated risk of bleeding. This highlights the importance of careful patient selection through the assessment of bleeding risk and comorbidities on a patient-by-patient basis.
The ULTIMA study is the first randomized clinical trial to demonstrate the superior efficacy of USAT over anticoagulation alone.9 In this study, which included a sample of 59 patients, the primary endpoint was the change in echocardiographic RV/LV ratio at 24 hours after treatment. In this study, which included only intermediate-risk patients with an RV/LV ratio ≥ 1, a greater reduction in the RV/LV ratio was observed in the USAT group compared to anticoagulation (0.30 ± 20 vs. 0.03 ± 16,
Another prospective study evaluating the safety and efficacy of USAT is the SEATTLE-II study.10 This study included a total of 150 patients from both intermediate and high-risk groups. The primary safety endpoint was major bleeding within 72 hours after the procedure, while the primary efficacy endpoint was the change in RV/LV ratio measured by CTPA at 48 hours. Regarding efficacy endpoints, similar to this study, positive results were observed in RV/LV ratio change, reduction in thrombus burden, and changes in PAP compared to before treatment. Regarding safety, major bleeding occurred in 14 patients (9.3%) within 72 hours, with no instances of intracranial hemorrhage. Although the definitions of major bleeding do not completely overlap with those in the study, both studies observed similar frequencies of major bleeding. In this study, intracranial hemorrhage was found in 4 patients (1.9%), whereas none were observed in the SEATTLE-II study. One reason for this difference could be the variation in bleeding risk profiles and comorbidities of the patients in the study.
Following these studies that demonstrated the efficacy and safety of USAT, another study aimed at determining the optimal tPA dose and infusion duration is the OPTALYSE-PE randomized clinical trial.11 In this study, 100 patients were randomized into 4 different groups with varying dose regimens. The efficacy endpoints were changes in RV/LV ratio and modified Miller score, while the safety endpoint was major bleeding within 72 hours after the procedure. Although similar results were obtained in terms of RV/LV change between the low-dose and high-dose groups, a significant reduction in thrombus burden was observed with increased dose. Increasing the tPA dose led to a reduction in thrombus burden without a corresponding increase in RV/LV change. It has been suggested that there is no linear relationship or correlation between PA embolic burden and RV dilatation. Additionally, the 1-year long-term follow-up of these patients demonstrated sustained benefits in terms of RV function, functional status, and quality of life.12 Regarding the safety endpoint, the major bleeding rate, defined similarly to this study, was 4% (including 1% intracranial), while the minor bleeding rate was 7%. Although the rate of major bleeding is similar to the study, the small sample size of the OPTALYSE-PE study and the fact that about one-fifth of the patients were from intermediate-low risk groups, along with stringent exclusion criteria, may mean that the bleeding events do not fully reflect real-world data for USAT treatment. Another limitation of the study is the lack of a control group receiving only anticoagulant therapy.
The KNOCOUT PE registry study is another investigation that addresses the limitations of previous studies, including OPTALYSE-PE, by examining safety endpoints and different dosing strategies with a larger patient cohort.13 This multicenter registry study, which included a total of 489 patients from only high and intermediate-to-high risk groups, has the primary efficacy endpoint as the change in RV/LV ratio, and the safety endpoint as the frequency of bleeding events defined similarly to the study. The mean tPA dose for all patients was reported as 18.1 ± 7.4 mg, and the mean infusion duration was 10.5 ± 5.37 hours, indicating that a lower dose and shorter duration of treatment were used compared to the study. However, the echocardiographic LV/RV ratio change was found to be 22.6%. The frequency of bleeding events was lower compared to previous studies, with major bleeding reported at 1.6% and intracranial bleeding at 0.9%. Another study comparing conventional CDT to anticoagulation in patients with intermediate-to-high risk acute PE is the CANARY randomized clinical trial.14 Due to a reduction in patient enrollment caused by disruptions during the COVID-19 pandemic, this study, which had lower statistical power, had a primary endpoint of the percentage of patients with an RV/LV ratio > 0.9 at 3 months of follow-up, and the safety endpoint was bleeding events. Due to the early termination of the study, no significant results were obtained for the primary endpoint. Regarding bleeding events, the CDT group of 46 patients experienced 1 major bleeding event and 3 minor bleeding events.
Although different dosing and infusion duration strategies have been used in USAT studies in the literature, lower doses and infusion durations have been preferred in these studies compared to the single-center results. However, in the single-center series of 225 patients, which includes all risk groups, it has been shown that bleeding events and mortality were not associated with increased tPA dose and infusion duration.15 Additionally, a linear relationship has been shown between increasing tPA doses and reduction in thrombus burden. In a meta-analysis by Kaymaz et al16 that evaluated results from 15 studies, the all-cause and cardiovascular mortality rates were reported as 3.2% and 2.2%, respectively, while the rates of major and minor bleeding were reported as 5.5% and 6.9%. To compare with STT, a meta-analysis comparing STT with anticoagulation reported a major bleeding rate of 9.2% in the STT group, whereas the study reported a rate of 5.9%.17
Study Limitations
Regarding the limitations of this study, although balance among variables was achieved using PSA methods, the study remains susceptible to bias due to operator-based treatment selection and its observational nature, making it less robust than results from a randomized controlled trial. Although a partially sufficient number of patients (n = 203) was reached for analysis in the treatment group, expanding the control group would lead to more accurate results from PSA methods. While the duration and dose of tPA administration vary from patient to patient in the USAT group, using a single treatment regimen could provide more definitive comparative results. One of the limitations of this study is that the treatment option in the group receiving only anticoagulant therapy was restricted to intravenous heparin, which prevented the comparison of different treatment modalities.
Another limitation is that follow-up CT was performed between 72 and 96 hours, leading to variations in timing between patients. Reviewing the literature, it is noteworthy that as the duration of follow-up imaging for evaluating the RV/LV ratio after treatment increases, the RV/LV ratios between the treatment and control groups tend to converge. This situation could impact one of the efficacy endpoints, specifically the RV/LV ratio and its changes. Furthermore, longer follow-up data could yield significant results concerning changes in the RV/LV ratio. The results of ongoing randomized clinical trials comparing USAT with anticoagulant therapy in acute PE patients17-
Conclusion
The quasi-experimental study based on real-world data has demonstrated that USAT with a moderate-dose, slow-infusion tPA regimen is superior to anticoagulant therapy alone in patients with acute PE at IHR, in terms of reduction in PA thrombus burden, improvement in RV dysfunction, and better clinical outcomes. However, it was associated with a slight increase in bleeding events. With insights from prospective studies on USAT treatment, evaluating bleeding risk on an individual basis and considering PE progression may justify personalizing tPA doses and infusion durations beyond standard protocols. This personalized approach could position USAT as a first-line treatment for IHR acute PE.
Supplementary Materials
Footnotes
References
- Konstantinides SV, Meyer G, Becattini C. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2020;41(4):543-603. https://doi.org/10.1093/eurheartj/ehz405
- Stevens SM, Woller SC, Kreuziger LB. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):e545-e608. https://doi.org/10.1016/j.chest.2021.07.055
- Finocchiaro S, Mauro MS, Rochira C. Percutaneous interventions for pulmonary embolism. EuroIntervention. 2024;20(7):e408-e424. https://doi.org/10.4244/EIJ-D-23-00895
- Kaymaz C, Tokgöz HC, Kültürsay B. Current insights for catheter-directed therapies in acute pulmonary embolism: systematic review and our single-center experience. Anatol J Cardiol. 2023;27(10):557-566. https://doi.org/10.14744/AnatolJCardiol.2023.3639
- Konstantinides SV, Torbicki A, Agnelli G. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3069a-3069k. https://doi.org/10.1093/eurheartj/ehu283
- Qanadli SD, El Hajjam M, Vieillard-Baron A. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415-1420. https://doi.org/10.2214/ajr.176.6.1761415
- Rudski LG, Lai WW, Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685-713. https://doi.org/10.1016/j.echo.2010.05.010
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. https://doi.org/10.1111/j.1538-7836.2005.01204.x
- Kucher N, Boekstegers P, Müller OJ. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479-486. https://doi.org/10.1161/CIRCULATIONAHA.113.005544
- Piazza G, Hohlfelder B, Jaff MR. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382-1392. https://doi.org/10.1016/j.jcin.2015.04.020
- Tapson VF, Sterling K, Jones N. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11(14):1401-1410. https://doi.org/10.1016/j.jcin.2018.04.008
- Piazza G, Sterling KM, Tapson VF. One-year echocardiographic, functional, and quality of life outcomes after ultrasound-facilitated catheter-based fibrinolysis for pulmonary embolism. Circ Cardiovasc Interv. 2020;13(8):e009012-. https://doi.org/10.1161/CIRCINTERVENTIONS.120.009012
- Sterling KM, Goldhaber SZ, Sharp ASP. Prospective multicenter international registry of ultrasound-facilitated catheter-directed thrombolysis in intermediate-high and high-risk pulmonary embolism (KNOCOUT PE). Circ Cardiovasc Interv. 2024;17(3):e013448-. https://doi.org/10.1161/CIRCINTERVENTIONS.123.013448
- Sadeghipour P, Jenab Y, Moosavi J. Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate-high–risk pulmonary embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol. 2022;7(12):1189-1197. https://doi.org/10.1001/jamacardio.2022.3591
- Kaymaz C, Akbal OY, Keskin B. An eight-year, single-center experience on ultrasound assisted thrombolysis with moderate-dose, slow-infusion regimen in pulmonary embolism. Curr Vasc Pharmacol. 2022;20(4):370-378. https://doi.org/10.2174/1570161120666220428095705
- Kaymaz C, Akbal OY, Tanboga IH. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: A meta-analysis. Curr Vasc Pharmacol. 2018;16(2):179-189. https://doi.org/10.2174/1570161115666170404122535
- Kjaergaard J. Low dose thrombolysis, ultrasound assisted thrombolysis or heparin for intermediate high risk pulmonary embolism. Clinicaltrials.gov. 2023;():-. https://clinicaltrials.gov/study/NCT04088292
- Klok FA, Piazza G, Sharp ASP. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: rationale and design of the HI-PEITHO study. Am Heart J. 2022;251():43-53. https://doi.org/10.1016/j.ahj.2022.05.011
- . Pulmonary embolism - thrombus removal with catheter-directed therapy. Clinicaltrials.gov. 2024;():-. https://clinicaltrials.gov/study/NCT05591118


