2Department of Cardiology, Faculty of Medicine, Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Türkiye
3Department of Medical Biology, Faculty of Medicine, University of Gaziantep, Gaziantep, Türkiye
4Department of Medical Pharmacology, Faculty of Medicine, Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Türkiye
5Department of Medical Services and Techniques, Kahramanmaraş Health Services Vocational School, Pathology Laboratory Techniques Pr., Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Türkiye
6Department of Cardiovascular Surgery, Faculty of Medicine, Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Türkiye
Abstract
Background: Arterial hypertension (HT) is a major risk factor for cardiovascular disease; however, its underlying mechanisms, particularly in primary HT, remain largely unclear. This knowledge gap has hindered the development of effective treatment strategies. Transient receptor potential (TRP) channels, which play a critical role in signal transduction, have been implicated in HT based on previous studies in cell culture and animal models. This study is the first study to analyze aortic tissue from patients with primary HT.
Methods: Ascending aortic tissue samples, reflecting central blood pressure (BP), were collected from patients with chronic HT (n = 59) and normotensive controls (n = 22) undergoing coronary artery bypass graft surgery. Transient receptor potential channel mRNA expression was analyzed using quantitative real-time polymerase chain reaction. Subgroup analyses were performed to assess the influence of demographic characteristics, biochemical parameters, antihypertensive medications, and coexisting cardiometabolic conditions on gene expression.
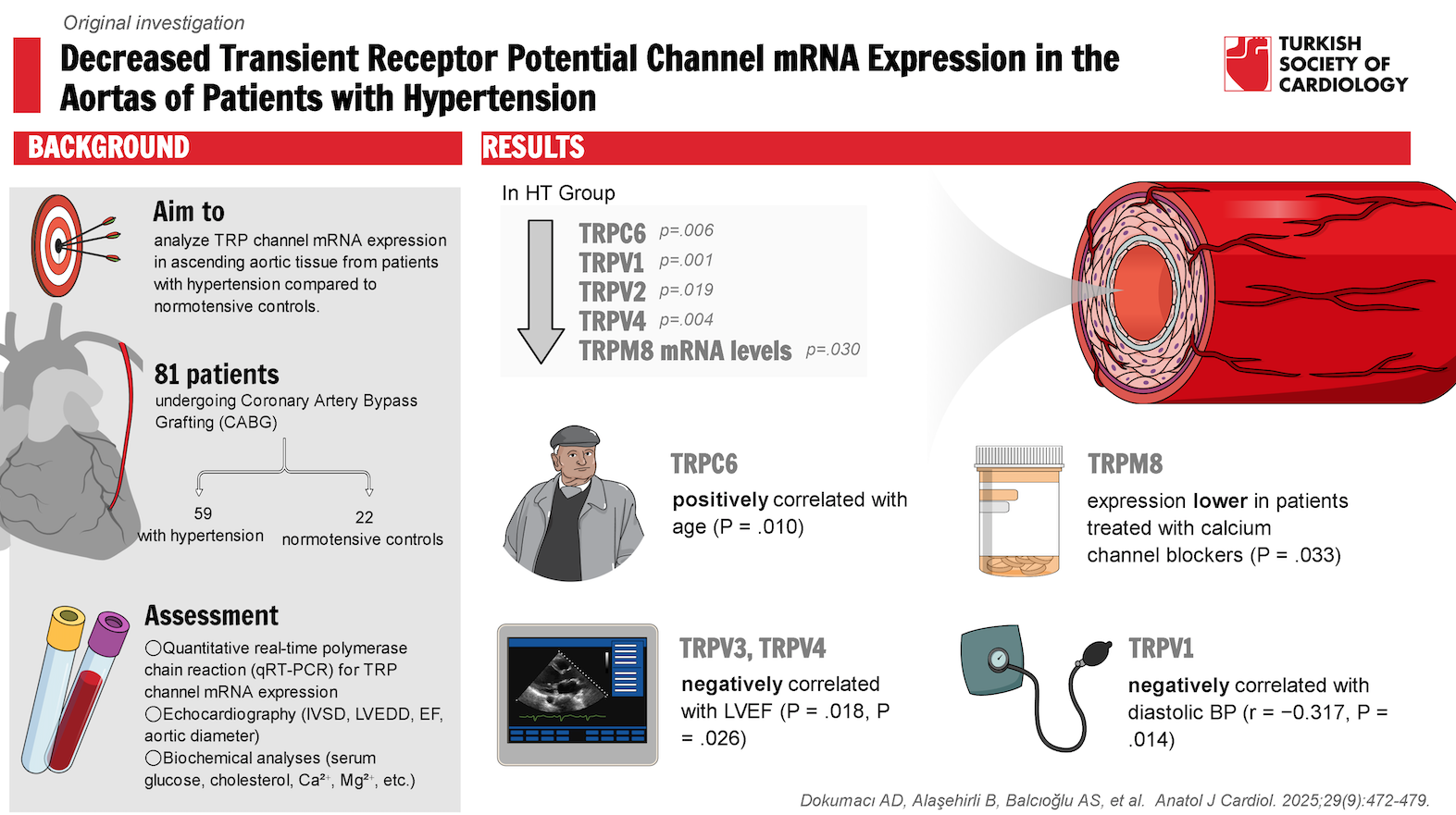
Results: Although no significant differences were observed in TRPC1 and TRPV3 mRNA expression between HT and control groups, TRPC6, TRPV1, TRPV2, TRPV4, and TRPM8 mRNA levels were significantly lower in patients with HT. TRPC6 expression showed a positive correlation with age, while TRPV1 expression demonstrated a negative correlation with diastolic BP.
Conclusion: These findings suggest that TRP channels could serve as potential therapeutic targets for HT and contribute to a better understanding of its pathogenesis.
Graphical Abstract
Highlights
- TRPC6, TRPV1/2/4, and TRPM8 are downregulated in the aortas of patients with HT.
- TRPC6 channel expression increases with age in the human aorta.
- Altered TRP channel expression may play a role in the pathogenesis of HT.
Introduction
Transient receptor potential (TRP) channels are a superfamily of ion channels expressed in various tissues and cells. They play essential roles in numerous physiological processes by responding to a wide range of stimuli, including changes in temperature, pH, ions (Ca2+ and Mg2+), hormones, inflammatory mediators, and oxidative stress.1 Transient receptor potential channels are classified into 6 subfamilies based on sequence homology: TRPC, TRPV, TRPM, TRPML, TRPA, and TRPP.2 These channels, widely expressed across different cell types, regulate ion homeostasis and influence intracellular signaling pathways, impacting both physiological and pathological processes.1
In the cardiovascular system, TRP channels are present in endothelial cells (ECs), vascular smooth muscle cells (VSMCs), inflammatory cells, and cardiomyocytes. They participate in various physiological processes, including G protein-coupled or tyrosine kinase receptor signaling, mechanosensation, vasoconstriction/vasodilation, proliferation, vascular permeability, and angiogenesis.1,
Several TRP channel subtypes help regulate vascular resistance and arterial pressure by modulating intracellular Ca2+ homeostasis and signaling, thereby significantly influencing vascular tone. Dysfunctional TRP channel activity can impair vascular function and may contribute to hypertension (HT).4 Changes in TRP channel expression have been linked to vascular dysfunction, remodeling, and inflammation in HT.1,
While previous studies have investigated the relationship between TRP channel expression and HT pathogenesis primarily in cell cultures and animal models no data are currently available on TRP channel expression in human aortic tissue from patients with HT.4,
In this study, the authors assessed the expression of TRP channel members (TRPC1, TRPC6, TRPV1, TRPV2, TRPV3, TRPV4, and TRPM8) in the ascending aortas of patients with HT and normotensive controls undergoing coronary artery bypass grafting (CABG) for severe coronary artery disease (CAD).
Methods
Patients and Methods
This study recruited 81 patients diagnosed with severe CAD who underwent CABG at the Cardiovascular Surgery Clinic between February 2021 and May 2022. A total of 64 male and 17 female patients were included in the study.
Patients were categorized into 2 groups based on their HT status. The HT group (n = 59) included patients with a history of HT and/or high BP, confirmed through BP measurements according to the European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines (
Among these patients, 53 were receiving at least 1 antihypertensive medication from various drug classes, while 6 were not undergoing any regular treatment for HT before surgery. The control group (n = 22) included patients with no prior history of HT or high BP. These patients maintained a systolic blood pressure (SBP) below 140 mmHg and a diastolic blood pressure (DBP) below 90 mmHg during both preoperative evaluations and hospital follow-up.
Of the 59 patients in the HT group, 34 had concurrent DM, 53 had dyslipidemia, and 22 had obesity. Among the 22 patients in the control group, 8 had DM, 18 had dyslipidemia, and 9 had obesity. Subgroup analyses were conducted based on comorbidities, including diabetes mellitus (DM), dyslipidemia, and obesity, in both the HT and control groups.
Exclusion Criteria
Patients under 18 years of age, pregnant individuals, and those with vasculitis, autoimmune diseases, severe kidney or liver disease, or a history of cancer were excluded from the study (
This study was approved by the Clinical Research Ethics Committee (Approval Date: January 27, 2021; Approval Number: 13). Written informed consent was obtained from all patients. No artificial intelligence technology was used in this study.
Echocardiography
Echocardiography was performed to evaluate left ventricular interventricular septum thickness (IVSD), left ventricular end-diastolic diameter (LVEDD), left ventricular ejection fraction (EF), and ascending aorta diameter in both groups.
Biochemical Analysis
Venous blood samples were collected from each patient following an overnight or at least 8-hour fasting period. Routine biochemical analyses were conducted using standart laboratory techniques at the Clinical Biochemistry Laboratory.
Human Aorta Samples
Ascending aorta tissue samples (2-3 mm) were collected from patients who met the inclusion criteria and provided written informed consent. These samples were obtained during the aortic incision for aortic cannulation during CABG. The tissue samples were stored at −20°C in RNA Save stabilization solution (Biological Industries, Israel) until analysis.
RNA Isolation and Real-Time Polymerase Chain Reaction
Total RNA was isolated from ascending aorta samples using NucleoZOL® (Macherey-Nagel GmbH & Co. KG, Germany) following the manufacturer’s protocol. RNA quantity and purity (260/280 nm ratio) were assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription was performed using the OneScript® Plus Reverse Transcriptase kit with an RNAse inhibitor (Applied Biological Materials, Canada). Quantitative real-time polymerase chain reaction (RT-PCR) was conducted using SYBR Green analysis (HOT FIREPol® SolisGreen® qPCR Mix 5x, Solis BioDyne, Estonia) on a QIAGEN Rotor Gene Q device (Corbett Research Pty Ltd, Australia), according to the manufacturer’s instructions. Primers for TRPC1, TRPC6, TRPV1-4, and TRPM8 (Macrogen, Türkiye) were validated to confirm the amplification of a single PCR product of the expected size (Supplementary Table 1). No signal was detected where reverse transcription was omitted. Gene expression levels were normalized to the endogenous reference gene beta-actin (ACTB) and analyzed using the 2−ΔΔCt method: ΔCt = CtTRP – CtACTB, where Ct represents the threshold cycle.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). The Shapiro-Wilk test was used to assess normality. The Student’s t-test was applied to normally distributed data, while the Mann–Whitney
Results
Demographic, Clinical, and Biochemical Characteristics of Patients
Transient Receptor Potential Channel Expression in Aortic Tissue
mRNA levels of TRPC6, TRPV1, TRPV2, TRPV4, and TRPM8 were significantly lower in the aortas of patients with HT compared to normotensive controls (
To further investigate the role of individual TRP channel isoforms in HT, gene expression was analyzed in subgroups defined by various clinical characteristics.
Discussion
This study provides the first human data on TRP channel gene expression in the aorta of patients with HT compared to normotensive controls undergoing CABG for severe CAD. The authors’ findings suggest that decreased mRNA levels of TRPC6, TRPV1, TRPV2, TRPV4, and TRPM8 in the aortas of patients with HT may contribute to HT pathogenesis.
TRPC1 is primarily expressed in VSMCs and ECs and in VSM contraction, endothelial-mediated vasodilation, proliferation, migration, and arterial remodeling.3,
TRPC6 is widely expressed in vascular tissues and plays a critical role in BP regulation.3 By mediating both receptor-activated and pressure-dependent increases in cytosolic Ca2+ in VSMCs, TRPC6 significantly contributes to vascular tone.12 Studies in TRPC6-
TRPV1 plays a key role in regulating vascular tone and arterial BP. Studies in rats have demonstrated that TRPV1 gene deletion increases BP, heightens salt sensitivity, worsens salt-induced HT, and exacerbates renal inflammatory responses and damage.20-
TRPV2 is involved in vasomotor regulation in response to mechanical stress and temperature changes.26,
TRPV3 is activated by temperature (>30°C) and specific ligands such as carvacrol and thymol and serves as a key regulator of vascular thermoregulatory mechanisms.28 However, no studies have previously examined its relationship with HT. In addition to observing a decreasing trend in TRPV3 expression in patients with HT, the authors found that TRPV3 expression was lower in patients with obesity and HT than in those with normal weight. Similar findings have been reported in previous studies, which demonstrated that TRPV3 activation promotes lipolysis and reduces diet-induced obesity.29 These results suggest that reduced TRPV3 expression may contribute to both obesity and obesity-related HT.
TRPV4 has been widely studied for its roles in vasodilation, vasoconstriction, vascular permeability, remodeling, and injury.30-
TRPM8 is a non-selective cation channel permeable to Ca2+, primarily recognized as a physiological sensor of environmental cold. TRPM8 activation influences vascular function and BP regulation.41 Consistent with the authors’ findings, experimental HT models have reported decreased TRPM8 expression.42,
Various studies have demonstrated that antihypertensive drugs can influence TRP channel expression.44-
In this study, most patients had DM, dyslipidemia, and obesity, along with HT. Notably, these diseases may influence TRP channel expression.48-
Additionally, the authors examined potential associations between TRP channel expression and comorbidities such as DM, obesity, and dyslipidemia. When the authors compared TRP channel expression in patients with and without DM, dyslipidemia, and obesity between the HT and control groups, the authors observed no significant differences based on these comorbidities. Nevertheless, the observed trend of decreased TRPC6 expression in patients with DM in the control group aligns with studies showing that high glucose levels downregulate TRPC6 expression.48 The negative correlation between TRPV1 expression and BMI in male patients with HT suggests a potential role for TRPV1 in obesity-related HT in males. This is consistent with findings demonstrating that TRPV1 deficiency exacerbates obesity-related HT by impairing mitochondrial Ca2+ homeostasis in brown adipose tissue.49
Given the regulatory role of cholesterol in TRP channel activity, the authors also investigated the relationship between serum total cholesterol levels and TRP channel expression.50 Despite most patients being on statin therapy for CAD, the authors observed positive correlations between total cholesterol levels and TRPV3, TRPV4, and TRPM8 expression, suggesting cholesterol’s regulatory effects of cholesterol on these channels. Additional studies with larger patient populations may further elucidate the impact of these comorbidities on TRP channel expression.
In summary, this study is particularly significant as it is the first to investigate TRP channel expression in human vascular tissue, specifically in the ascending aorta, which best reflects central BP. The authors’ findings suggest that altered TRP channel expression may play a critical role in HT pathogenesis.
Study Limitations
First, this study included a relatively small number of normotensive patients, particularly female patients, undergoing CABG for severe CAD compared to the HT group. Second, variations in cardiovascular and other medications used in both groups may have influenced TRP gene expression to different extents. Third, due to the limited tissue sample size, the authors were only able to analyze mRNA expression rather than protein expression, which provides functional insights. Fourth, baseline data showed that 89.1% of patients with HT were receiving antihypertensive drugs, making it difficult to isolate the direct effects of HT on TRP expression. Fifth, environmental factors such as stress, air pollution, diet, and tissue collection time can affect gene expression, and these variables were not controlled for in this study. Lastly, analyzing the entire vascular tissue prevented us from distinguishing VSM-specific and endothelium-specific changes in gene expression, limiting the authors’ understanding of the specific cell types involved in TRP channel dysregulation in HT.
Future large-scale clinical studies could provide deeper insights into the changes in TRP channel expression and function in HT, as well as the underlying mechanisms. This may ultimately contribute to the development of more effective treatment strategies for HT.
Conclusion
In conclusion, the authors’ findings indicate downregulation of TRP channel gene expression (TRPC6, TRPV1, TRPV2, TRPV4, and TRPM8) in ascending aortic tissue from patients with HT. Notably, TRPC6 expression increased with age in the HT group. These findings suggest that TRP channels may serve as promising therapeutic targets for HT prevention and treatment. This study paves the way for developing more effective strategies for HT management. Further research is needed to fully elucidate the role of TRP channels in HT pathogenesis.
Supplementary Materials
Footnotes
References
- Rios FJ, Sarafian RD, Camargo LL, Montezano AC, Touyz RM. Recent advances in understanding the mechanistic role of transient receptor potential ion channels in patients with hypertension. Can J Cardiol. 2023;39(12):1859-1873.
- Rather MA, Khan A, Wang L. TRP channels: role in neurodegenerative diseases and therapeutic targets. Heliyon. 2023;9(6):e16910-.
- Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95(2):645-690.
- Liu D, Xiong S, Zhu Z. Imbalance and dysfunction of transient receptor potential channels contribute to the pathogenesis of hypertension. Sci China Life Sci. 2014;57(8):818-825.
- Zhu Z, Xiong S, Li Q. The role of transient receptor potential channels in hypertension and metabolic vascular damage. Exp Physiol. 2016;101(11):1338-1344.
- Jesus RLC, Araujo FA, Alves QL, Dourado KC, Silva DF. Targeting temperature-sensitive transient receptor potential channels in hypertension: far beyond the perception of hot and cold. J Hypertens. 2023;41(9):1351-1370.
- Ma J, Yang L, Ma Y, Wang X, Ren J, Yang J. Targeting transient receptor potential channels in cardiometabolic diseases and myocardial ischemia reperfusion injury. Curr Drug Targets. 2017;18(15):1733-1745.
- Martín-Bórnez M, Galeano-Otero I, Del Toro RD, Smani T. TRPC and TRPV channels’ role in vascular remodeling and disease. Int J Mol Sci. 2020;21(17):6125-.
- Lin XH, Hong HS, Zou GR, Chen LL. Upregulation of TRPC1/6 may be involved in arterial remodeling in rat. J Surg Res. 2015;195(1):334-343.
- Noorani MMZ, Noel RC, Marrelli SP. Upregulated TRPC3 and downregulated TRPC1 channel expression during hypertension is associated with increased vascular contractility in rat. Front Physiol. 2011;2():42-.
- Chen X, Yang D, Ma S. Increased rhythmicity in hypertensive arterial smooth muscle is linked to transient receptor potential canonical channels. J Cell Mol Med. 2010;14(10):2483-2494.
- Abdinghoff J, Servello D, Jacobs T, Beckmann A, Tschernig T. Evaluation of the presence of TRPC6 channels in human vessels: a pilot study using immunohistochemistry. Biomed Rep. 2022;16(5):42-.
- Dietrich A, Mederos Y Schnitzler MMY, Gollasch M. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25(16):6980-6989.
- Bae YM, Kim A, Lee YJ. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25(4):809-817.
- Liu Y, Thilo F, Kreutz R. Tissue expression of TRPC3 and TRPC6 in hypertensive Munich Wistar Frömter rats showing proteinuria. Am J Nephrol. 2010;31(1):36-44.
- Liu DY, Thilo F, Scholze A. Increased store-operated and 1-oleoyl-2-acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. J Hypertens. 2007;25(4):799-808.
- Harraz OF, Jensen LJ. Vascular calcium signalling and ageing. J Physiol. 2021;599(24):5361-5377.
- Baghaiee B, Bayatmakoo R, Karimi P, Shannon Pescatello LS. Moderate aerobic training inhibits middle-aged induced cardiac calcineurin-NFAT signaling by improving TGF-ß, NPR-A in wistar rats. Cell J. 2021;23(7):756-762.
- Erac Y, Selli C, Kosova B, Akcali KC, Tosun M. Expression levels of TRPC1 and TRPC6 ion channels are reciprocally altered in aging rat aorta: implications for age-related vasospastic disorders. Age (Dordr). 2010;32(2):223-230.
- Yu SQ, Ma S, Wang DH. Activation of TRPV1-expressing renal sensory nerves of rats with n-oleoyldopamine attenuates high-fat-diet-induced impairment of renal function. Int J Mol Sci. 2023;24(7):6207-.
- Yu SQ, Ma S, Wang DH. Ablation of TRPV1-positive nerves exacerbates salt-induced hypertension and tissue injury in rats after renal ischemia-reperfusion via infiltration of macrophages. Clin Exp Hypertens. 2021;43(3):254-262.
- Wang Y, Wang DH. Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension. Am J Physiol Ren Physiol. 2009;297(6):F1550-F1559.
- Wang Y, Babánková D, Huang J, Swain GM, Wang DH. Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate-salt hypertension. Hypertension. 2008;52(2):264-270.
- Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47(3):609-614.
- Mohammadifard N, Moazeni F, Azizian-Farsani FA. Genetic variation in salt taste receptors impact salt intake and blood pressure. Sci Rep. 2023;13(1):4037-.
- Perálvarez-Marín A, Solé M, Serrano J. Evidence for the involvement of TRPV2 channels in the modulation of vascular tone in the mouse aorta. Life Sci. 2024;336():122286-.
- Iwata Y, Ito S, Wakabayashi S, Kitakaze M. TRPV2 channel as a possible drug target for the treatment of heart failure. Lab Invest. 2020;100(2):207-217.
- Fromy B, Josset-Lamaugarny A, Aimond G. Disruption of TRPV3 impairs heat-evoked vasodilation and thermoregulation: a critical role of CGRP. J Invest Dermatol. 2018;138(3):688-696.
- Hu Y, Zou W, Zhang L. TRPV3 facilitates lipolysis and attenuates diet-induced obesity via activation of the NRF2/FSP1 signaling axis. Free Radic Biol Med. 2024;221():155-168.
- Mao A, He D, Zhang K. Functional role of coupling the endothelial TRPV4 and KCa 3.1 channels in regulating coronary vascular tone. Br J Pharmacol. 2023;180(17):2266-2279.
- McFarland SJ, Weber DS, Choi CS, Lin MT, Taylor MS. Ablation of endothelial TRPV4 channels alters the dynamic Ca2+ signaling profile in mouse carotid arteries. Int J Mol Sci. 2020;21(6):2179-.
- Zhu Y, Chu Y, Wang S. Vascular smooth muscle TRPV4 (transient receptor potential vanilloid family member 4) channels regulate vasoconstriction and blood pressure in obesity. Hypertension. 2023;80(4):757-770.
- Daneva Z, Kuppusamy M. TRPV4-dependent signaling mechanisms in systemic and pulmonary vasculature. Curr Top Membr. 2022;89():1-41.
- Geng L, Zhang C, He C. Physiological levels of fluid shear stress modulate vascular function through TRPV4 sparklets. Acta Biochim Biophys Sin (Shanghai). 2022;54(9):1268-1277.
- Seki T, Goto K, Kiyohara K. Downregulation of endothelial transient receptor potential vanilloid type 4 channel and small-conductance of Ca2+-activated K+ channels underpins impaired endothelium-dependent hyperpolarization in hypertension. Hypertension. 2017;69(1):143-153.
- Diaz-Otero JM, Yen TC, Ahmad A. Transient receptor potential vanilloid 4 channels are important regulators of parenchymal arteriole dilation and cognitive function. Microcirculation. 2019;26(6):e12535-.
- Sonkusare SK, Dalsgaard T, Bonev AD. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal. 2014;7(333):ra66-.
- Gao F, Wang DH. Impairment in function and expression of transient receptor potential vanilloid type 4 in Dahl Salt-Sensitive Rats: Significance and Mechanism. Hypertension. 2010;5(4):1018-1025.
- Onishi M, Yamanaka K, Miyamoto Y, Waki H, Gouraud S. Trpv4 involvement in the sex differences in blood pressure regulation in spontaneously hypertensive rats. Physiol Genomics. 2018;50(4):272-286.
- Chambers LC, Yen M, Jackson WF, Dorrance AM. Female mice are protected from impaired parenchymal arteriolar TRPV4 function and impaired cognition in hypertension. Am J Physiol Heart Circ Physiol. 2023;324(5):H581-H597.
- Izquierdo C, Martín-Martínez M, Gómez-Monterrey I, González-Muñiz R. TRPM8 channels: advances in structural studies and pharmacological modulation. Int J Mol Sci. 2021;22(16):8502-.
- Huang F, Ni M, Zhang JM, Li DJ, Shen FM. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol Med Rep. 2017;15(4):1900-1908.
- Voronova IP, Khramova GM, Evtushenko AA, Kozyreva TV. Effect of skin ion channel TRPM8 activation by cold and menthol on thermoregulation and the expression of genes of thermosensitive TRP ion channels in the hypothalamus of hypertensive rats. Int J Mol Sci. 2022;23(11):6088-.
- Zhou Y, Yan H, Li T, Xie M, Li X, Zhao C. New use of old medicine: nifedipine acts on the TRP family and inflammatory proteins in the treatment of chilblain. Burns. 2022;48(2):372-380.
- Li W, Chen X, Riley AM. Long-term spironolactone treatment reduces coronary TRPC expression, vasoconstriction, and atherosclerosis in metabolic syndrome pigs. Basic Res Cardiol. 2017;112(5):54-.
- Chi-Xianggeng C, Hu-Bo H, Yu SY. Losartan treating podocyte injury induced by Ang II via downregulation of TRPC6 in podocytes. J Renin Angiotensin Aldosterone Syst. 2015;16(4):1118-1124.
- Liang M, Zhong W, Miao F, Wu H, Liu Y. Effects of losartan on vasomotor function and canonical transient receptor potential channels in the aortas of sinoaortic denervation rats. Clin Exp Hypertens. 2018;40(1):39-48.
- Graham S, Ding M, Sours-Brothers S, Yorio T, Ma J-X, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Ren Physiol. 2007;293(4):F1381-F1390.
- Li L, Ma L, Luo Z. Lack of TRPV1 aggravates obesity-associated hypertension through the disturbance of mitochondrial Ca2+ homeostasis in brown adipose tissue. Hypertens Res. 2022;45(5):789-801.
- Rosenbaum T, Morales-Lázaro SL. Regulation of ThermoTRP channels by PIP2 and cholesterol. Adv Exp Med Biol. 2023;1422():245-277.


