2Department of Cardiovascular Medicine, Beijing Anzhen Hospital, Capital Medical University and National Clinical Research Center for Cardiovascular Diseases, Beijing, China
Abstract
Background: Patients with hypertrophic obstructive cardiomyopathy (HOCM) have few available nonsurgical treatment options. The feasibility of CARTOSound-guided catheter radiofrequency ablation (RFA) has been reported previously; however, relevant data are limited. The objective is to retrospectively evaluate the effectiveness and safety of CARTOSound-guided RFA for patients with HOCM.
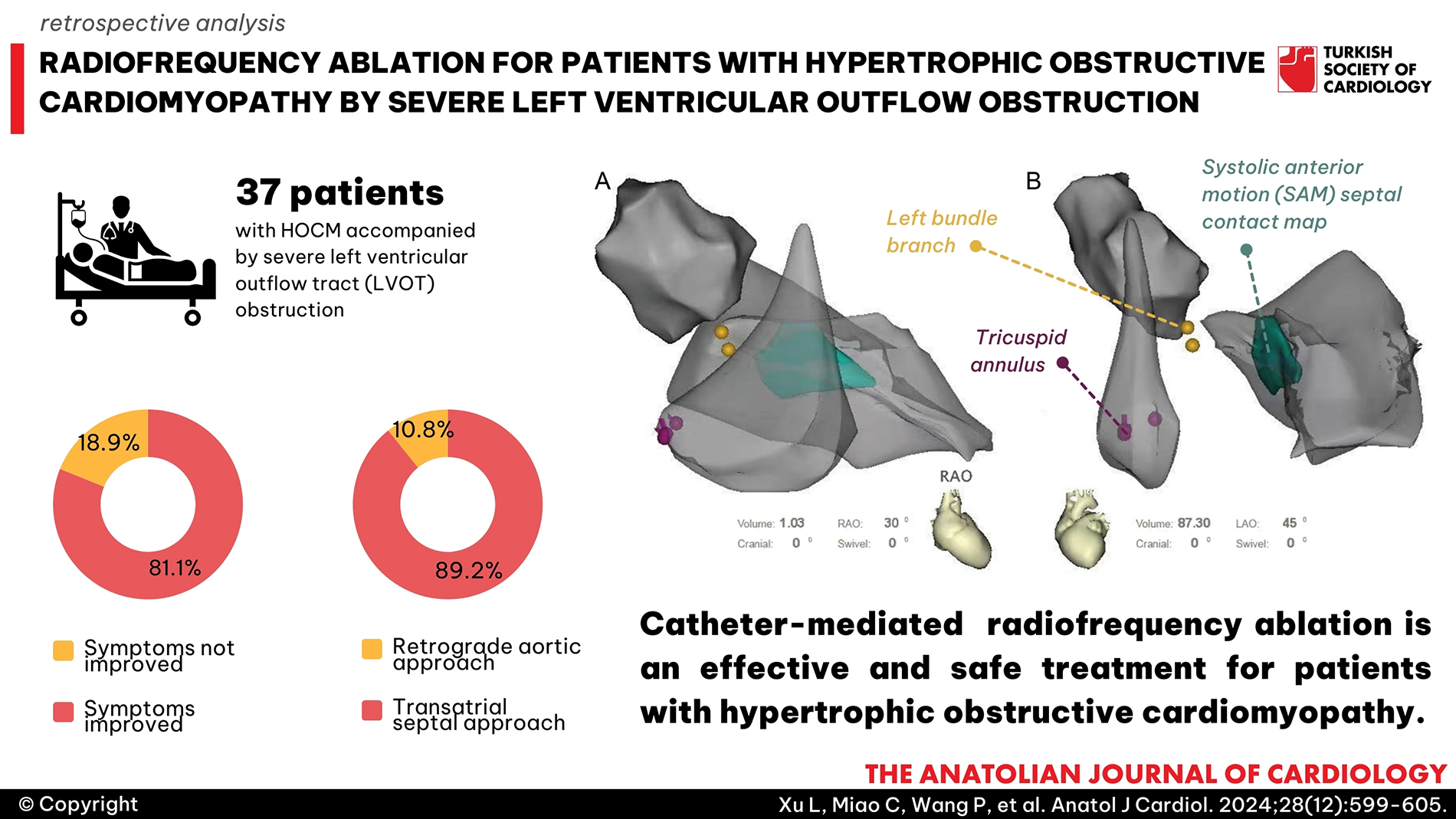
Methods: Thirty-seven patients with successive HOCM accompanied by severe left ventricular outflow tract (LVOT) obstruction underwent CARTOSound-guided RFA were reviewed. The intracardiac echocardiography (ICE) images obtained were merged with the CARTO system to create a shell of the left ventricle. The systolic anterior motion-septal contact area marked from the ICE images was considered the target area for the current delivery of RFA. Follow-up data of the LVOT gradient examined before, 1 month, 6 months, 1 year, and every year after catheter-mediated RFA were accessed.
Results: The symptoms of 30 patients (81.1%) improved during the follow-up after RFA. The symptoms of all 30 patients were alleviated from the New York Heart Association (NYHA) class IV/III/II to the NYHA class II/I. A sustained and significant gradient reduction was observed in 28 patients (75.7%). The invasive pressure gradient of LVOT was 84.43 ± 27.55 mm Hg before RFA and 42.78 ± 36.38 mm Hg after RFA (P < .001), with a decrease of 41.65 ± 19.72 mm Hg. The median drop in pressure gradient was 36.0% (1.0-67.0%).
Conclusions: Catheter-mediated RFA is an effective and safe treatment for patients with HOCM. However, its long-term efficacy and safety should be validated in the future by conducting multicenter clinical trials with large sample sizes.
Graphical Abstract
Highlights
- Radiofrequency ablation is an effective and safe treatment for patients with hypertrophic obstructive cardiomyopathy.
- The trans-atrial septal approach is a key factor in achieving the efficacy and safety of the procedure because of the stability of the catheter and the good tissue-catheter contact force.
- Due to the stability of the catheter and the relatively low ablation power, no serious complications occurred.
Introduction
Hypertrophic obstructive cardiomyopathy (HOCM) is a genetic disease that increases the left ventricular outflow tract (LVOT) gradient (LVOTG). It is an autosomal dominant hereditary disease with varying penetrance.1,
The evidence suggests that symptomatic patients who do not experience relief from medical therapy may benefit from septal myectomy and alcohol septal ablation (ASA) to improve hemodynamics and clinical symptoms.7-
CARTOSound system (Biosense Webster, Diamond Bar, CA, USA) is a technology that integrates both an electroanatomic electrophysiology mapping system to guide and mark ablation lesions and intracardiac echocardiography (ICE) images to provide high quality images.14 CARTOSound system could provide the precise RFA target area, and thus progressively improve the success rate of the procedure.14 However, the data on the effectiveness and safety of CARTOSound-guided RFA for patients with HOCM are limited. This study aimed to summarize our experience of using CARTOSound-guided RFA for HOCM.
Methods
Study Population
The patients diagnosed as symptomatic HOCM who received the CARTOSound (Biosense Webster, Diamond Bar, CA, USA)-guided RFA in our hospital from December 2018 to June, 2023 were retrospectively analyzed. The inclusion criteria were 1) patients with symptomatic HOCM who refused to undergo myectomy and ASA; 2) those who had a resting or provoked LVOTG ≥50 mm Hg associated with remarkable SAM; and 3) patients with drug-refractory symptoms (New York Heart Association (NYHA) class III or IV). Hypertrophic obstructive cardiomyopathy patients without outflow tract obstruction or incomplete data were excluded. The study procedures were approved by the Ethics Committee of our institution.
Evaluation of LVOTG
All patients underwent resting or provoked echocardiography (Phillips IE33 scanner, Phillips S5-1 probe) before RFA procedures. During the procedures, the pressure gradient of the LVOT was measured by inserting a pigtail catheter at apical and subaortic sites while the patient was at rest. The procedures were performed only when the gradient met the obstruction criterion. If the gradient did not meet the obstruction criterion (≥ 50 mm Hg), isoproterenol was administered to provoke the pressure gradient in the patient.
Establishment of a 3-Dimensional Anatomical Model
The SoundStar catheter (Biosense Webster, Diamond Bar, CA, USA) was inserted through the left femoral vein to access the right atrium and right ventricle (RV). The phased array probe was used to generate ICE images of the tricuspid annulus, RV, and left ventricle (LV), and the resulting images were merged with the CARTO system (Biosense Webster, Diamond Bar, CA, USA). Structural borders were manually contoured and reconstructed at the end of diastole. Next, a new map of the multiplanar contact area of the anterior MV leaflet and the hypertrophied septum during systole (SAM-septal contact area,
Ablation by the Retrograde Aortic or Trans-Septal Approach
Once the ablation catheter was inserted in the LV, the patient was administered heparin (100 U/kg) intravenously to maintain an activated clotting time between 300 seconds and 350 seconds. The His bundle locations and left bundle branch potentials were directly mapped and annotated on the CARTO shell with respect to the SAM-septal contact area. The NaviStar THERMOCOOL catheter (Biosense Webster, Diamond Bar, CA, USA) or the THERMOCOOL SMARTTOUCH catheter (Biosense Webster, Diamond Bar, CA, USA) was used for RFA as per the choice of the operator. The procedures were performed on the first 4 patients using the retrograde aortic access approach after the specialized conduction system was mapped on the LV septum. If there was difficulty in maintaining catheter stability and tissue-catheter contact force (CF) due to the dynamic septum and turbulent LVOT blood flow with the retrograde aortic access, the transatrial septal approach was used with the assistance of a steerable sheath (Agilis, St Jude Medical, St Paul, Minnesota, USA) with a large or medium curvature, which improved catheter stability and CF. Radiofrequency ablation energy lesions were placed over the SAM-septal contact area using the CARTO and ICE navigation combination (
Acute and Long-Term Success
The acute success of RFA was achieved with an invasive gradient reduction of 50%. The long-term success was defined as the LVOTG being reduced to less than 50 mm Hg.
Follow-Up
To prevent a paradoxical increase in obstruction (PIO), all patients were administered beta blockers and/or calcium channel blockers after the procedure. Additionally, methylprednisolone sodium succinate was administered intravenously to avoid tissue edema at the end of the procedure. All patients were followed up by performing transthoracic echocardiography (TTE) at baseline, 1 month, 6 months, and every year thereafter. The documentation of the physical examination, 12-lead electrocardiogram, and TTE was reviewed. Echocardiography results at the last follow-up were considered to determine the long-term success of the procedure.
Statistical Analysis
Continuous variables were presented as means ± standard deviations or medians and interquartile ranges. Groups were compared using the
Statement
DeepL was adopted for linguistic editing.
Results
Baseline Data
Thirty-seven patients were included in this study, including 17 males and 20 females. Their ages ranged from 34 to 77 years, with a mean age of 55.0 ± 10.0 years. The baseline characteristics and complicated diseases are listed in
Intracardiac Echocardiography-Guided RFA
Left ventricular ablation was performed on 33 (89.2%) patients using the transatrial septal approach, whereas the remaining 4 (10.8%) patients were treated using the retrograde aortic approach. The ablation area was 4.40 cm2 (2.75-6.45 cm2). The invasive pressure gradient of LVOT was 84.43 ± 27.55 mm Hg before RFA and 42.78 ± 36.38 mm Hg after RFA (
Transthoracic Echocardiography Assessment and Follow-Up
The patients were followed up for 6-64 months, with a median follow-up of 36 months. They were followed up at 1 month, 6 months, and 12 months after RFA and every year thereafter. No patients were lost to follow-up.
The IVS before and after RFA was 20.27 ± 4.45 mm and 18.32 ± 4.10 mm, respectively (
The line chart presented the TTE pressure gradient before and after the procedure, at 1 month, 6 months, 1 year, and every year thereafter, with the mean level shown in the bar chart (
The K–M curves of the results are presented in
Complications
Nine patients developed a complete LBBB during the ablation, 10 patients developed a left posterior fascicle block, and 2 patients developed a left anterior fascicle block. Nonetheless, no patient showed acute pericardial tamponade or PIO during the ablation.
Discussion
For patients with drug-resistant, symptomatic HOCM, surgical myectomy is the most commonly used treatment approach.17 However, surgical myectomy is morbid and its success rate depends on the experience of operators. Alcohol septal ablation is an alternative non-surgical approach widely applied to patients who refuse to undergo surgery or are at high risk for surgery.18,
The radiofrequency-generated energy was effective in improving the NYHA class and LVOTG in most patients in the present study. Thirty patients (81.1%) were alleviated from the NYHA class IV/III/II to the NYHA class II/I, and 28 patients (75.7%) were observed with sustained and significant gradient reduction. These results were similar to a previous study.23 The efficacy of a procedure depends on 2 main aspects. First, the use of an intracardiac ultrasound catheter allows for precise ablation during the procedure. A study has shown that ICE is an effective tool during the procedure because even mild damage can interrupt the SAM-septal feedback mechanism, thereby effectively reducing LVOTGs.14 Tissue injury sites and locations are equally important, and real-time ICE images help operators precisely target the SAM-septal contact area and confirm tissue edema at the desired location on the septum. Although the tissue damage size may appear smaller compared to other forms of septal reduction, its accuracy is sufficient to affect the SAM and eventually reduce LVOTGs. Second, increasing the lesion size is a major goal of catheter-mediated ablation for HOCM. When RF duration and power are constant, lesion depth, diameter, and size can be increased proportionately with increasing CF.
Left ventricular ablation was performed on 33 (89.2%) patients using the transatrial septal approach, whereas the remaining 4 (10.8%) patients were treated using the retrograde aortic approach. During ablation, there was a risk of His bundle damage owing to catheter instability. To prevent the catheter from jumping to the His bundle, we adopted the transatrial septal approach instead of the retrograde aortic access approach. The transatrial septal approach improves the stability of the catheter, making the catheter tissue CF sufficient. Catheter stability is crucial for ensuring the effectiveness and duration of a single ablation. Herein, no cases of AV block, cardiac tamponade, or PIO were observed in the 37 patients, which indicated the transatrial septal approach has fewer complications and improves the catheter attachment pressure and effectiveness.25
Ventricular ectopies originated from the mechanical effect of the catheter interrupted AV conduction observation. In this condition, the coronary sinus catheter was paced to confirm the intact AV conduction. Furthermore, to prevent cardiac tamponade induction, we manipulated the mapping and ablation catheters gently with the help of a fluoroscopy and 3‐dimensional mapping system. Since outflow tract obstruction is the result of basal septal hypertrophy and SAM of the MV, we established an ultrasonic anatomical model to accurately mark the position of mitral valve and interventricular septum adhesion. The 3‐dimensional mapping system enables us to perform precise intervention, thus reducing the pressure stage difference and alleviating symptoms. Moreover, cardiac effusion was monitored by intracardiac echocardiology at any time. In contrast to previous results,13,
Study Limitations
The feasibility of CARTOSound-guided catheterRFA for HOCM has been reported in recent years. However, the sample size is always small, and follow-up time is generally short. Our preliminary results further suggest RFA as an alternative treatment in HOCM patients, especially in those who are unsuitable for ASA or surgical myectomy. The present study has several limitations that need to be acknowledged. First, Lawrenz and Kuhn26 first reported the use of RFA by RV septal aspect in a 45-year-old patient with severe HOCM. Lawrenz et al16 reported no significant difference in the LVOTG after ablation between the LV aspect and the RV septal aspect (
Conclusions
Catheter-mediated RFA achieved an immediate decrease in the catheter pullback gradient and a further reduction of the gradient during follow-up. However, the long-term efficacy and safety of the procedure need to be validated by conducting multicenter prospective randomized controlled clinical trials with large sample sizes.
Footnotes
References
- Richard P, Charron P, Carrier L. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227-2232. https://doi.org/10.1161/01.CIR.0000066323.15244.54
- Bos JM, Will ML, Gersh BJ, Kruisselbrink TM, Ommen SR, Ackerman MJ. Characterization of a phenotype-based genetic test prediction score for unrelated patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2014;89(6):727-737. https://doi.org/10.1016/j.mayocp.2014.01.025
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. https://doi.org/10.1016/j.jacc.2015.01.019
- Batzner A, Schäfers HJ, Borisov KV, Seggewiß H. Hypertrophic obstructive cardiomyopathy. Dtsch Arztebl Int. 2019;116(4):47-53. https://doi.org/10.3238/arztebl.2019.0047
- Valdigem BP, Correia EB, Moreira DAR. Septal ablation with radiofrequency catheters guided by echocardiography for treatment of patients with obstructive hypertrophic cardiomyopathy: initial experience. Arq Bras Cardiol. 2022;118(5):861-872. https://doi.org/10.36660/abc.20200732
- Maron MS, Olivotto I, Betocchi S. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. https://doi.org/10.1056/NEJMoa021332
- Cho YH, Quintana E, Schaff HV. Residual and recurrent gradients after septal myectomy for hypertrophic cardiomyopathy-mechanisms of obstruction and outcomes of reoperation. J Thorac Cardiovasc Surg. 2014;148(3):909-915. https://doi.org/10.1016/j.jtcvs.2014.05.028
- Smedira NG, Lytle BW, Lever HM. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85(1):127-133. https://doi.org/10.1016/j.athoracsur.2007.07.063
- Fernandes VL, Nielsen C, Nagueh SF. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc Interv. 2008;1(5):561-570. https://doi.org/10.1016/j.jcin.2008.07.005
- Veselka J, Jensen MK, Liebregts M. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016;37(19):1517-1523. https://doi.org/10.1093/eurheartj/ehv693
- Kuhn H, Lawrenz T, Lieder F. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol. 2008;97(4):234-243. https://doi.org/10.1007/s00392-007-0616-7
- Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin Res Cardiol. 2007;96(12):864-873. https://doi.org/10.1007/s00392-007-0578-9
- Sreeram N, Emmel M, de Giovanni JV. Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J Am Coll Cardiol. 2011;58(24):2501-2510. https://doi.org/10.1016/j.jacc.2011.09.020
- Cooper RM, Shahzad A, Hasleton J. Radiofrequency ablation of the interventricular septum to treat outflow tract gradients in hypertrophic obstructive cardiomyopathy: a novel use of CARTOSound(R) technology to guide ablation. Europace. 2016;18(1):113-120. https://doi.org/10.1093/europace/euv302
- Crossen K, Jones M, Erikson C. Radiofrequency septal reduction in symptomatic hypertrophic obstructive cardiomyopathy. Heart Rhythm. 2016;13(9):1885-1890. https://doi.org/10.1016/j.hrthm.2016.04.018
- Lawrenz T, Lawin D, Radke K, Stellbrink C. Acute and chronic effects of endocardial radiofrequency ablation of septal hypertrophy in HOCM. J Cardiovasc Electrophysiol. 2021;32(10):2617-2624. https://doi.org/10.1111/jce.15203
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet. 2017;389(10075):1253-1267. https://doi.org/10.1016/S0140-6736(16)31321-6
- Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: the future of treatment. Eur J Heart Fail. 2020;22(2):228-240. https://doi.org/10.1002/ejhf.1715
- Ommen SR, Mital S, Burke MA. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2020;142(25):e533-e557. https://doi.org/10.1161/CIR.0000000000000938
- Rigopoulos AG, Sakellaropoulos S, Ali M. Transcatheter septal ablation in hypertrophic obstructive cardiomyopathy: a technical guide and review of published results. Heart Fail Rev. 2018;23(6):907-917. https://doi.org/10.1007/s10741-018-9706-z
- Cooper RM, Shahzad A, McShane J, Stables RH. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: safe and apparently efficacious but does reporting of aggregate outcomes hide less-favorable results, experienced by a substantial proportion of patients?. J Invasive Cardiol. 2015;27(7):301-308.
- Steggerda RC, Balt JC, Damman K, van den Berg MP, Ten Berg JM. Predictors of outcome after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Special interest for the septal coronary anatomy. Neth Heart J. 2013;21(11):504-509. https://doi.org/10.1007/s12471-013-0453-4
- Liu Q, Qiu H, Jiang R. Selective interventricular septal radiofrequency ablation in patients with hypertrophic obstructive cardiomyopathy: who can benefit?. Front Cardiovasc Med. 2021;8():-. https://doi.org/10.3389/fcvm.2021.743044
- Poon SS, Cooper RM, Gupta D. Endocardial radiofrequency septal ablation - A new option for non-surgical septal reduction in patients with hypertrophic obstructive cardiomyopathy (HOCM)?: A systematic review of clinical studies. Int J Cardiol. 2016;222():772-774. https://doi.org/10.1016/j.ijcard.2016.08.123
- Li X, Liu T, Cui B, Chen Y, Tang C, Wu G. Radiofrequency catheter septal ablation via a trans-atrial septal approach guided by intracardiac echocardiography in hypertrophic obstructive cardiomyopathy: one-year follow-up. RCM. Rev Cardiovasc Med. 2024;25(2):38-. https://doi.org/10.31083/j.rcm2502038
- Lawrenz T, Kuhn H. Endocardial radiofrequency ablation of septal hypertrophy. A new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol. 2004;93(6):493-499. https://doi.org/10.1007/s00392-004-0097-x


