Abstract
The landscape of metabolic health has evolved dramatically in recent years. Once viewed as a collection of distinct conditions, disorders like obesity, type 2 diabetes, cardiovascular disease, chronic kidney disease, and fatty liver disease are now understood to share common pathophysiological roots. This review explores the transition from the traditional concept of metabolic syndrome toward more comprehensive and clinically relevant frameworks, namely, the cardiovascular–kidney–metabolic (CKM) syndrome proposed by the American Heart Association, and the systemic metabolic disorder (SMD) framework introduced by the European Atherosclerosis Society. Both models recognize the progressive, multisystemic nature of metabolic dysfunction, highlight the need for stage-based risk stratification, and emphasize early intervention. The authors discuss the central role of dysfunctional adiposity and ectopic fat in driving organ-specific damage, and examine the growing body of evidence supporting the use of novel therapies such as sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RA), and gastric inhibitory polypeptide (GIP)/GLP-1RA in delivering multiorgan protection. By comparing the CKM and SMD models, the authors highlight their complementary nature and shared call for a shift in clinical thinking away from isolated management and toward integrated, multidisciplinary care. As the burden of metabolic dysfunction continues to rise, the need to recognize obesity as a chronic disease and to develop practical, collaborative strategies across cardiology, nephrology, endocrinology, and hepatology becomes even more urgent. The rise of cardiometabolic medicine offers a timely and necessary response to this growing challenge, creating opportunities for better prevention, earlier detection, and improved outcomes for the patients.
Graphical Abstract
Highlights
- Metabolic disorders affect multiple organs and progress through overlapping stages.
- The CKM syndrome and SMD frameworks offer updated, clinically useful approaches to risk stratification.
- Dysfunctional adiposity and ectopic fat are central drivers of systemic organ damage.
- Novel therapies such as SGLT2 inhibitors and GLP-1RAs offer multiorgan protection.
- A shift toward integrated, multidisciplinary care defines the rise of cardiometabolic medicine.
Introduction
Adult obesity rates have doubled globally since 1990, with current estimates exceeding 1 billion affected individuals worldwide.1 In recent years, the number of people affected by cardiometabolic diseases has also increased significantly. This trend has slowed or even reversed the improvements we had seen in cardiovascular disease (CVD) outcomes in past decades.2 Data from the Global Burden of Disease study show that the number of people living with CVD almost doubled between 1990 and 2019.3 In the United States, more than 1 in 4 adults had been shown to have at least one of the cardiac, renal, and metabolic conditions according to data from 2015 to 2020.4
Once considered as distinct entities, conditions such as obesity, type 2 diabetes mellitus (DM), atherosclerotic CVD, heart failure (HF), chronic kidney disease (CKD), and metabolic dysfunction-associated steatotic liver disease (MASLD) are now recognized as interconnected disorders that share common pathophysiological pathways.5 Many concepts were proposed to define the shared mechanisms. The concept of “cardiorenal syndrome” illustrates the bidirectional relationship between heart and kidney dysfunction, while “metabolic syndrome” or “cardiometabolic syndrome” highlights the close association between excess adiposity, metabolic risk, and cardiovascular outcomes.6-
To reflect this broader picture, new terms have been proposed. In 2023, the American Heart Association (AHA) introduced the term cardiovascular-kidney-metabolic (CKM) syndrome, emphasizing the cumulative burden of metabolic dysfunction on cardiovascular and renal system. A staging model was proposed to capture the pathophysiological continuum, delineate risk categories, and inform appropriate preventive and therapeutic approaches.11 Yet, the liver’s involvement remained underemphasized in this model. Metabolic dysfunction-associated steatotic liver disease reflects the hepatic aspect of metabolic dysfunction and functions both as a driver and a result of the systemic metabolic derangement.12 Recently, European Atherosclerosis Society (EAS) suggested a new clinical staging term, systemic metabolic disorder (SMD), to describe and guide the management of the group of metabolic dysfunction-related diseases, involving cardiovascular, renal, and hepatic systems.10 While obesity remains a major driver, other organ-specific defects, such as insulin resistance originating in skeletal muscle or impaired lipid handling in the liver, can also initiate the cascade of systemic dysfunction and inflammation. These shifts in nomenclature reflect a deeper understanding of disease biology and help for further advances in prevention and treatment strategies.
This review explores how the understanding of metabolic disease has changed over time, examining the move from the old concept of metabolic syndrome to CKM syndrome and SMD. The key pathophysiological mechanisms, clinical features, and novel treatment strategies that are relevant in this new framework, with a focus on prevention, early detection and risk assessment strategies are highlighted. Future approaches, strategies for better systemic integration of care and the emergence of cardiometabolic medicine as a new subspecialty, are also discussed.
Pathophysiological Continuum: From Cardiometabolic Risk to Multisystemic Dysfunction
The systemic effects of excess adiposity was first noted in the 1960s through the findings from the Framingham Heart Study; however, it was in 1988 when Gerald Reaven formally introduced the concept that a cluster of common metabolic abnormalities, namely insulin resistance, hypertension, and a dyslipidemic profile characterized by elevated triglycerides and low HDL cholesterol, could collectively increase the risk of premature CVD. He termed this cluster “Syndrome X.”13 The central role of insulin resistance in cardiometabolic pathology was highlightened, independent of overt hyperglycemia or type 2 DM.
In 2001, the National Cholesterol Education Program—Third Adult Treatment Panel (NCEP-ATP III) introduced a practical framework for identifying individuals at high cardiometabolic risk using simple, accessible clinical markers. They created 5 diagnostic criteria that could be applied in everyday clinical practice. First of all, abdominal obesity, particularly visceral adiposity, was highlighted as the most prevalent and clinically relevant manifestation and waist circumference (WC) was adopted as a surrogate marker for abdominal fat accumulation. Other 4 additional parameters were; fasting triglycerides, high-density lipoprotein (HDL) cholesterol, blood pressure, and fasting glucose levels. The panel proposed the more inclusive term “metabolic syndrome” to describe this constellation of interrelated risk factors and it was defined by the presence of at least 3 out of 5 criteria.14 Despite its relevance to both clinical practice and public health, the concept of metabolic syndrome has long been the subject of debate.15 Controversies have centered around its underlying pathophysiology, the validity of its diagnostic criteria, and its limitations as a risk stratification tool.16 Critics have argued that the binary nature of the diagnosis fails to reflect disease severity or capture the full spectrum of cardiometabolic risk.17
Visceral Adiposity and Ectopic Fat Deposition as Central Drivers of the Pathophysiology
Obesity results from a complex, multifactorial process that leads to sustained positive energy balance and, ultimately, to the accumulation of lipids within adipocytes. As adiposity progresses, the storage and mobilization capacity of subcutaneous adipose tissue becomes limited. Main key predictor of cardiometabolic risk in individuals with overweight or obesity is the presence of excess visceral adipose tissue.18 It refers to hypertrophic fat accumulation within the abdominal cavity, particularly along structures such as the greater omentum and mesentery. This pattern is frequently associated with ectopic triglyceride deposition in non-adipose tissues, including the liver,skeletal muscle,pancreas, and renal sinus, as well as intrapericardial,mediastinal, and even intramyocardial compartments.19 The preferential storage of lipids in visceral depots, rather than in more metabolically safe peripheral or subcutaneous compartments, is increasingly recognized as a sign of adipose tissue dysfunction.20 It is characterized by a range of structural and functional abnormalities, including impaired adipogenesis,resistance to insulin-mediated suppression of lipolysis,reduced fatty acid uptake, and excessive collagen deposition within the extracellular matrix. These changes are often accompanied by chronic low-grade inflammation, driven by immune cell infiltration and proinflammatory cytokine release, as well as altered vascular architecture and remodeling.21 Together, they contribute to systemic metabolic disturbances that underlie the progression of metabolic syndrome and its transition into multisystem organ dysfunction.
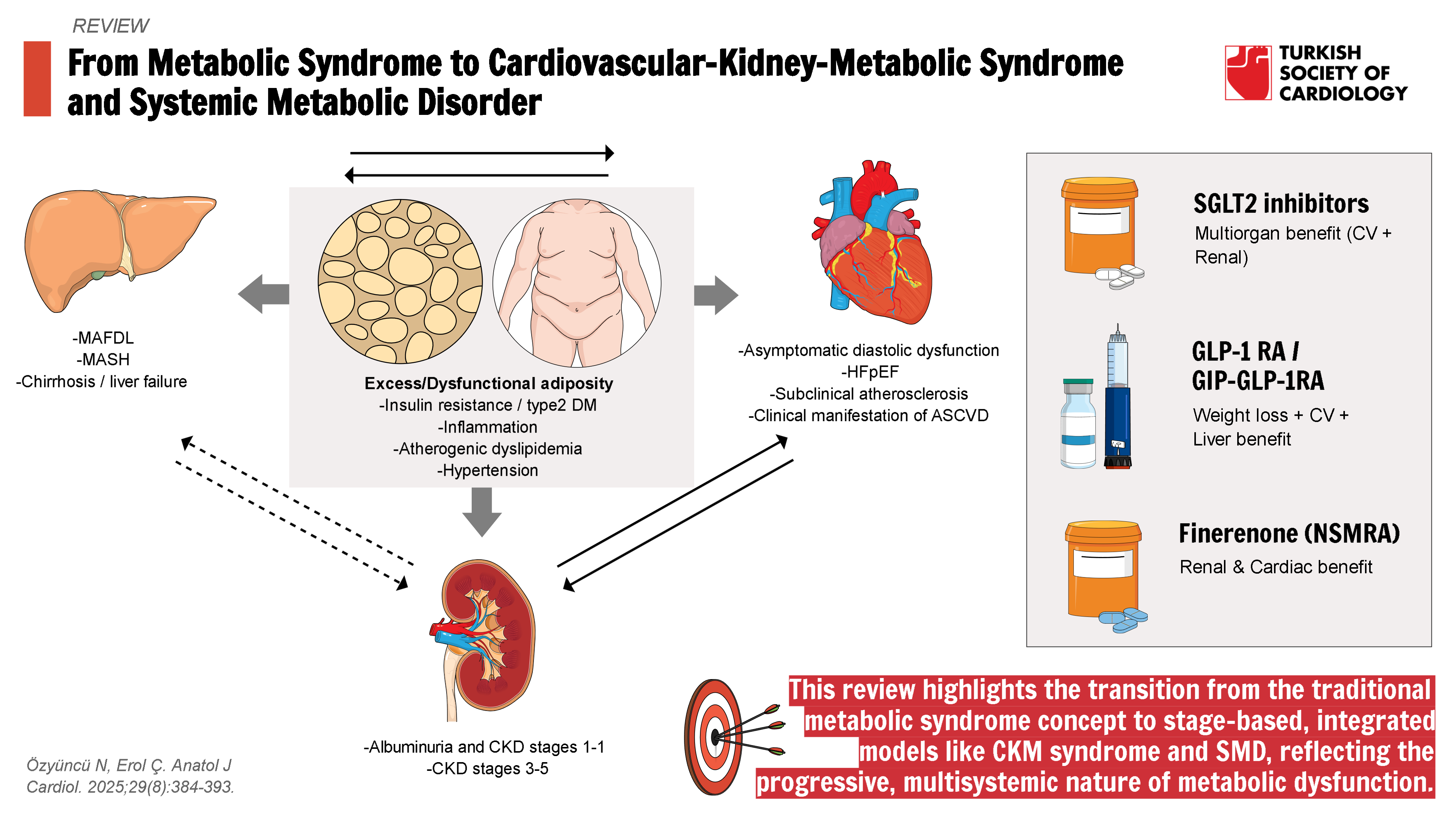
Ectopic fat accumulation is a hallmark of metabolic dysregulation and leads to a phenomenon known as lipotoxicity,a toxic overload of lipids in non-adipose tissues. Lipotoxicity provokes organ-specific fibro-inflammatory responses, which vary based on the location and extent of lipid deposition. These responses influence each individual’s metabolic phenotype, shaped by factors such as the severity of cellular injury, the resilience of affected tissues, and the balance between inflammatory and fibrotic signaling.22 Ultimately, this process contributes to the development of systemic metabolic dysfunction and multi-organ damage, including insulin resistance, atherosclerosis, cardiac remodeling, hepatic inflammation, and renal impairment. The role of excess or dysfunctional adiposity in driving multiorgan dysfunction, primarily affecting the cardiovascular, renal, and hepatic systems, is illustrated in
Novel Metabolic Therapies Targeting Multiorgan Protection: Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-Like Peptide-1 Receptor Agonists, and Gastric-Inhibitory Polypeptide/ Co-Agonists
The world has been facing an obesity pandemics with marked rising of interconnected diseases, such as DM, hypertension, dyslipidemia, and HF, especially the HF with preserved ejection fraction (HFpEF). In the past 5 years, the field of cardiometabolic medicine has undergone a notable shift in both understanding and clinical practice. Conditions that were once managed separately, such as obesity, type 2 DM, CKD, atherosclerotic CVD, HF, and MASLD, are now viewed as interconnected expressions of underlying metabolic disturbance. They are now recognized as parts of a shared pathophysiological network, where each affected organ contributes to and amplifies systemic inflammation, hormonal imbalance, and metabolic dysfunction.23 The cumulative effect is a self-perpetuating cycle that drives disease progression, worsens prognosis, and negatively impacts quality of life.
As the understanding of cardiometabolic diseases has evolved, 2 therapeutic classes, SGLT2 inhibitors and GLP-1RAs, have emerged as transformative options. Initially developed for glycemic control in patients with type 2 DM, these agents have demonstrated a wide range of benefits beyond glucose lowering. Sodium-glucose cotransporter 2 inhibitor use in type 2 DM revealed significant improvements in cardiovascular outcomes. Regardless of the presence of diabetes, empaglifozin and dapaglifozin improved outcomes in HF and CKD patients.24-
Shared organ-protective mechanisms suggested for SGLT2 inhibitors and GLP-1RAs are anti-oxidant, anti-inflammatory, and anti-fibrotic effects, enhancement of myocardial energetics, decrease in neurohormonal activation, improvement in endothelial function, promotion of vasodilation, reduction in arterial stiffness, and improvement in renal function.23 Multiorgan protective effects of these drugs bridged the gap between specialties like cardiology, endocrinology, nephrology, and hepatology. Understanding the systemic metabolic disease concept supported a more integrated and coordinated approach to care, instead of treating each organ system separately.
Although not primarily classified as a metabolic agent, a NSMRA, finerenone has demonstrated significant benefits in patients with HFpEF and diabetic kidney disease.38,
The Growing Obesity Crisis and the Emergence of Systemic Metabolic Concepts
With the growing global burden of obesity and weight gain, the cardiovascular community has encountered an increasing number of patients presenting with complex, multisystemic metabolic disturbances. In response, a recent consensus statement has been published addressing the clinical implications of obesity and cardiometabolic medicine has been proposed as a novel specialty for this systemic dysfunction.40,
Over the past 5 years, there has been growing recognition of the detrimental effects of excess adipose tissue and a marked shift toward more integrated approaches in the management of metabolic syndrome and its related multi-organ dysfunction.42 The 2021 European Society of Cardiology (ESC) guideline on CVD prevention regarded the patients with moderate-to-severe CKD, as high and very high CVD risk.43 However, this qualitative approach overlooks the opportunity to personalize cardiovascular preventive therapies by incorporating quantitative markers of CKD. A recently developed approach, termed the “CKD Add-on,” enables the incorporation of CKD markers, estimated glomerular filtration rate (eGFR) and albuminuria, into existing cardiovascular risk prediction models.44 It is validated that this Add-ons with CKD parameters improved the risk prediction of CVD beyond SCORE2 and SCORE2-OP.45
In 2023, AHA released a presidential advisory addressing the growing burden of poor cardiovascular-kidney and metabolic health and introduced the concept of CKM syndrome. As will be discussed in the following section, the advisory proposed a 4-stage classification of the syndrome and introduced a novel cardiovascular risk assessment model known as PREVENT.11 The interactions between metabolic syndrome, CVD and CKD leading to multi-organ damage with high adverse cardiovascular events were defined as a systemic disorder. It was an important call to action to optimize the systemic metabolic health problem in the population, indicating the requirement for interdisciplinary care and integrated management starting from childhood.
In parallel with the emergence of CKM syndrome, growing attention has also been directed toward the hepatic manifestation of metabolic dysfunction. In 2023, the redefinition of non-alcoholic fatty liver disease (NAFLD) as MASLD marked a major shift in terminology, emphasizing its pathophysiological links to metabolic disorders rather than exclusionary alcohol criteria.46 Metabolic dysfunction-associated steatotic liver disease frequently coexists with other features of systemic metabolic dysfunction, including obesity, insulin resistance, hypertension, dyslipidemia, and type 2 DM and is increasingly recognized as both a consequence and a contributor to multisystemic disease.16 Metabolic dysfunction-associated steatotic liver disease also has a bidirectional relationship with the development and progression of CKM syndrome. To better reflect the complex interplay among the heart, kidneys, liver, and metabolic pathways, an expanded concept, cardiovascular–renal–hepatic–metabolic (CRHM) syndrome, has been proposed.12 This broader framework encourages an integrated diagnostic approach that aims to slow disease progression and reduce the risk of further organ damage.
Building on these insights, in the early 2025, EAS released a consensus statement and introduced the concept of SMD, to describe the constellation of metabolic abnormalities that disrupt the function of multiple organ systems.10 Recognizing these conditions as interconnected, rather than isolated disorders, and incorporating the liver into the model promotes a more comprehensive strategy for clinical management and may contribute meaningfully to addressing the growing burden of cardiometabolic disease.
In the following section, the authors will provide a more detailed overview of both the CKM syndrome and the SMD framework, focusing on their definitions, staging systems, and clinical implications.
Defining Cardiovascular–Kidney–Metabolic Syndrome and Systemic Metabolic Disorder
As understanding of metabolic diseases has advanced, it has become increasingly clear that cardiovascular, renal, hepatic, and metabolic dysfunctions often coexist and interact in a bidirectional manner. This recognition has prompted the development of new conceptual frameworks to better describe the complex, multisystem nature of these conditions. Two such approaches, CKM syndrome, proposed by the AHA, and SMD, introduced by the EAS, aim to move beyond isolated risk factor models and provide structured staging systems based on shared pathophysiological mechanisms. These models not only facilitate more accurate risk stratification, but also support more integrated and targeted clinical management strategies.
Cardiovascular–Kidney–Metabolic Syndrome
The concept of CKM syndrome emphasizes the interconnected pathophysiology linking cardiovascular, renal, and metabolic systems, areas traditionally approached as separate clinical domains.11 There is a 4-stage classification system to stratify patients according to their level of risk and degree of organ involvement. Stage 1 includes individuals at increased risk due to genetic predisposition or lifestyle-related factors. Stage 2 comprises those with established cardiometabolic risk factors such as obesity, hypertension, dyslipidemia, or insulin resistance/DM. In Stage 3, early signs of organ dysfunction become evident, including subclinical cardiac or renal abnormalities. Stage 4 represents individuals with overt CVD, HF, or CKD. The definitions of the stages are summarized in
Screening and Management Strategies for the Cardiovascular–Kidney–Metabolic Stages
The CKM staging model enables clinicians to stratify individuals according to the severity of their metabolic risk, allowing for timely preventive interventions aimed at delaying or reversing disease progression. A key focus within this framework is the early identification of individuals in the preclinical phase, prior to the onset of overt cardiovascular or renal disease. Cardiovascular disease begins developing early in life and risk factor exposure during childhood and adolescence has been shown to contribute to early cardiovascular abnormalities, many of which persist into adult life.
The screening strategy for CKM syndrome is structured across 2 life stages: early life (<21 years) and adulthood (≥21 years), emphasizing timely identification of metabolic risk factors and subclinical disease. In early life, annual screening includes weight and height, blood pressure (starting at age 3), and mental and behavioral health assessments. Fasting lipid profiles are recommended between ages 9-11 and again between 17 years and 21 years, especially in children with a family history of CVD or lipid disorders. Additional testing with fasting plasma glucose and hemoglobin A1c (HbA1c) is advised for children with obesity or other risk factors. In adulthood, screening expands to include social determinants of health, BMI, and WC. Annual screening for metabolic syndrome components is recommended for individuals with stage 2 CKM or higher. For those at earlier stages, screening intervals vary between 2 years and 5 years depending on the degree of risk. Additional evaluations include non-invasive liver fibrosis assessment (e.g., FIB-4), urine albumin-creatinine ratio (UACR) for CKD staging, and calcium scoring for atherosclerotic CVD risk refinement. Subclinical HF screening using echocardiography and/or biomarkers may also be considered, although precise recommendations are still evolving.11
The management of CKM syndrome is staged and progressively adapted to disease severity, ranging from excess adiposity to established CVD. There is now strong evidence supporting the use of antihyperglycemic agents such as SGLT2 inhibitors and GLP-1RAs in reducing cardiovascular events and slowing the progression of CKD. Despite these benefits, their utilization in clinical practice remains limited. Barriers include clinician prescribing habits and systemic issues such as high out-of-pocket costs and formulary restrictions, which limit patient access.47,
Below, the authors summarize the proposed management strategies for each CKM syndrome stage:11
Stage 1: Excess or dysfunctional adiposity
Stage 2: Established CKM risk factors:
Stage 3: Subclinical CVD in CKM syndrome:
Stage 4: Established CVD in CKM syndrome:
Proposed Novel Risk Calculation Tool: PREVENT
Epidemiological studies have consistently demonstrated strong associations between CKM-related markers, such as impaired kidney function and metabolic dysregulation, and the risk of total CVD, especially atherosclerotic CVD and HF. There has been a continuous effort to refine cardiovascular risk assessment beyond traditional risk factors, leading to search for novel markers that can enhance predictive accuracy. American Heart Association proposed a novel cardiovascular risk estimation tool, the PREVENT risk model, that incorporates markers of kidney function and metabolic health, providing a more comprehensive assessment than traditional models.50
The PREVENT risk equations were developed to estimate the likelihood of total CVD in adults without pre-existing CVD and provide risk predictions over both 10- and 30-year time. Notably, these models include also HF as a formal endpoint and use age as the time scale. The base PREVENT model integrates traditional cardiovascular risk factors (blood pressure, HDL and total cholesterol, diabetes status, BMI, and tobacco use) along with kidney function (eGFR). Medication use is also incorporated into the base model, with antihypertensive and statin therapies as separate predictive components. Additional versions of the model allow for further risk stratification in high-risk populations by incorporating UACR or HbA1c where available, especially for individuals with baseline CKD or diabetes. Another version includes social determinants of health through indices such as the social deprivation index, supporting more individualized prediction.50
This sex-specific risk model for adults aged 30-79 years, represents an important advancement by including eGFR as a standard variable, recognizing HF as a key outcome, and excluding race from the predictive algorithm. Globally, CKD is often diagnosed at a relatively advanced stage and by recommending the routine assessment of both eGFR and UACR, this new score system may help early identification of CKD and timely initiation of interventions aimed at slowing disease progression.51 The modular design also permits optional inclusion of kidney, metabolic, and social health variables, allowing for personalized and risk-adapted decision-making in CKM care. Importantly, the use of absolute risk assessment to guide the initiation and intensity of preventive strategies can now be applied to HF. Given the rising burden of HF related morbidity and mortality, identifying individuals at risk has become increasingly important and current evidence strongly supports that HF is largely preventable in clinical practice.52
Systemic Metabolic Disorder
While the CKM syndrome framework has brought clarity to the interconnected nature of cardiovascular, renal, and metabolic health, it does not fully cover the broader spectrum of metabolic dysfunction, particularly the role of the liver. Recognizing this, the EAS recently introduced the term SMD to capture the complex, multi-organ impact of metabolic dysregulation. Systemic metabolic disorder emphasizes the progressive, systemic nature of metabolic abnormalities, which often begin silently and manifest across dysfunctional adiposity playing a central pathogenic role.10 This concept broadens our perspective beyond traditional risk clusters, encouraging earlier recognition, stratification, and intervention across the continuum of metabolic disease. At its core, SMD is closely linked to dysfunctional adiposity as a central feature; however, it can also originate from primary metabolic defects in organs such as skeletal muscle or the liver. In muscle, impaired insulin sensitivity shifts energy substrates toward fat and liver tissue, whereas hepatic vulnerability may disturb lipid handling and disrupt nutrient fluxes systemically. These imbalances gradually impair metabolic function across several organs, giving rise to a state of multisystemic dysfunction. To better understand and manage SMD, experts have proposed a practical staging system.10 This model is based on how the disease develops and gives doctors an easy way to track its progression and decide when to start treatment.
Systemic metabolic disorder includes several key features that may vary in severity, particularly in its early phases, before advancing toward widespread multi-organ dysfunction. The staging system incorporates the main systemic manifestations of SMD, such as insulin resistance or prediabetes, type 2 DM, MASLD, hypertension, atherogenic dyslipidemia, chronic inflammation, HF, and kidney disease. There are 3 stages in SMD. Stage 1 is marked by metabolic abnormalities without evident organ damage. Stage 2 involves early signs of organ involvement and stage 3 reflects established and more advanced organ damage. The specific criteria for each stage of SMD are outlined in the
Using this newly proposed staging system, researchers analyzed data from the UK Biobank to estimate the prevalence and outcomes of SMD Stages 1 and 2 among European adults aged 40-69 years. Stage 1 was identified in 58% of participants, most commonly characterized by overweight and dyslipidemia, with additional features such as liver steatosis and hypertension. Only 18% had pre-diabetes, though true insulin resistance rates may be higher. Stage 2 was found in 19% of the population and showed a predominance of subclinical atherosclerosis and CKD, with fewer individuals presenting with MASH, asymptomatic diastolic dysfunction, or type 2 DM. Notably, organ damage in Stage 2 was often limited to a single organ, possibly due to individual susceptibility. Stage 1 likely reflects an earlier, reversible phase of metabolic disruption, while Stage 2 signals inflammatory progression and breakdown of compensatory mechanisms. Long-term follow-up revealed a stepwise increase in all-cause mortality risk: 6% in Stage 1 and 49% in Stage 2, confirming the clinical relevance and predictive value of the staging system.10
Management of Systemic Metabolic Disorder
The primary aim in the clinical management of SMD is to prevent disease progression and minimize the risk of end-organ damage. Achieving this requires a personalized treatment approach, with lifestyle modification remaining the cornerstone of care. Stage-specific management strategies for this complex, multi-organ condition are outlined below:10
Stage 1: Early metabolic disturbance:
Stage 2: Subclinical organ involvement:
Stage 3: Clinical organ damage:
Systemic Metabolic Disorder and Cardiovascular–Kidney–Metabolic Syndrome: Two Sides of the Same Metabolic Coin
Recent international statements have highlighted the urgent need for a more refined approach to the diagnosis and management of metabolic disorders, given the global rise in obesity and related complications. Both the EAS consensus on SMD and the AHA’s framework for CKM syndrome emphasize early identification, a stepwise staging approach, and the importance of recognizing metabolic dysfunction as a progressive, multisystem process. There is agreement that BMI alone is insufficient for assessing metabolic health and should be complemented by additional measures such as WC, tailored for ethnicity. Both statements stress the need for early, interdisciplinary screening for metabolic risk factors, particularly in individuals with excess adiposity, before organ damage occurs. Clinical management in both approaches is structured around disease staging, beginning with lifestyle interventions and progressively incorporating risk factor evaluation and therapeutic intensification as the condition advances. This convergence supports a holistic, proactive approach that aims to prevent progression and reduce long-term complications.
Future Directions
Despite the progress in understanding and managing cardiometabolic disorders through CKM syndrome and SMD models, many challenges remain. Globally, there is an urgent need to recognize that obesity is formally defined as a chronic disease state, rather than a lifestyle condition. We need to ensure it receives the appropriate clinical attention and resources it demands.
One key need is detecting early-stage metabolic dysfunction before significant organ damage develops. Current screening tools often miss people at the first stages, especially if they do not yet have DM or high blood pressure. Expanding the use of anthropometric measures, such as WC alongside BMI, may improve the identification and management of excess adiposity. Also, using simple and accessible tests, such as UACR, HbA1c, liver fibrosis scoring, and echocardiography, can help to identify early signs of organ involvement.
A second need is improving risk prediction using more personalized tools. Genetic testing, molecular markers, and newer approaches like metabolomics and microbiome analysis could help to identify who is at greater risk for progression.54,
Another barrier is limited access to effective medications. Treatments like SGLT2 inhibitors, GLP-1RA, and finerenone show strong protective effects across organs, but they are underused due to cost and policy restrictions. Making these therapies more available should be a public health priority.
There is also a need for global agreement on definitions and staging. While CKM syndrome highlights heart, kidney, and metabolic health, the SMD concept includes the liver and emphasizes early, systemic changes. Both models recognize the progressive nature of metabolic dysfunction and the need for early intervention. Unifying these frameworks with a shared language and strategy would support better implementation worldwide.
Importantly, care must become more collaborative. Patients with SMD or CKM often have overlapping problems that require input from cardiologists, nephrologists, endocrinologists, hepatologists, and primary care providers. Interdisciplinary care teams and integrated clinical pathways are needed to ensure effective and consistent management at every stage. These patients mostly die from cardiovascular complications driven by the cumulative impact of these risk factors and this underscores the need for a broader perspective in cardiovascular medicine. The ESC is actively committed to advancing cardiometabolic cardiovascular medicine to improve outcomes in this growing and vulnerable patient population.41
Conclusion
Over the past decades, the understanding of metabolic diseases has evolved significantly. What was once broadly defined as “metabolic syndrome” is now recognized as a complex, progressive, and multisystem disorder that affects not just the cardiovascular system but also the kidneys, liver, and other organs. This shift in perspective has led to new clinical frameworks, CKM syndrome and SMD, that reflect this broader and more integrated view. Both models acknowledge the shared pathophysiology behind conditions like type 2 DM, hypertension, atherosclerosis, and fatty liver disease, and offer a structured, stage-based approach to guide risk assessment and management.
Importantly, these conditions rarely exist in isolation. Most patients present with overlapping dysfunction across multiple organs, and the majority ultimately experience adverse cardiovascular outcomes. This highlights the urgent need to move beyond traditional, isolated treatment models. As such, a shift toward cardiometabolic medicine, bringing together expertise mainly from cardiology, but also nephrology, hepatology, and endocrinology, is not only logical but necessary. We also need more accessible tools for early detection, wider availability of effective treatments like SGLT2 inhibitors and GLP-RA, and a global consensus on staging and terminology.
In this new era, managing metabolic disease means thinking systemically and acting early. Recognizing SMD and CKM as progressive, interconnected syndromes is a crucial step forward to intervene sooner, treat more effectively, and ultimately improve outcomes for a growing number of patients at risk.
Footnotes
References
- . Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403(10431):1027-1050.
- Nikolaou M, Theodorakis N, Feretzakis G. Nationwide mortality trends from 2001 to 2020 in Greece: health policy implications under the scope of aging societies. Hellenic J Cardiol. 2024;():S1109-9666(24)00177-5-.
- Roth GA, Mensah GA, Johnson CO. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021.
- Ostrominski JW, Arnold SV, Butler J. Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999-2020. JAMA Cardiol. 2023;8(11):1050-1060.
- Angelidi AM, Sanoudou D, Hill MA, Mantzoros CS. Management of patients with the cardio renal liver metabolic syndrome: the need for a multidisciplinary approach in research, education and practice. Metabolism. 2024;159():155997-.
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527-1539.
- Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33-45.
- Oğuz A, Kılıçkap M, Güleç S. Risk of cardiovascular events in patients with metabolic syndrome: results of a population-based prospective cohort study (PURE Turkey). Anatol J Cardiol. 2020;24(3):192-200.
- Krentz A, Jacob S, Heiss C. Rising to the challenge of cardio-renal-metabolic disease in the 21st century: translating evidence into best clinical practice to prevent and manage atherosclerosis. Atherosclerosis. 2024;396():118528-.
- Romeo S, Vidal-Puig A, Husain M. Clinical staging to guide management of metabolic disorders and their sequelae: a European Atherosclerosis Society consensus statement. Eur Heart J. 2025;():ehaf431-.
- Ndumele CE, Rangaswami J, Chow SL. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606-1635.
- Theodorakis N, Nikolaou M. From cardiovascular-kidney-metabolic syndrome to cardiovascular-renal-hepatic-metabolic syndrome: proposing an expanded framework. Biomolecules. 2025;15(2):213-.
- Reaven GM. Why syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1(1):9-14.
- . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III). JAMA. 2001;285(19):2486-2497.
- Dağdelen S, Yildirim T, Erbaş T. Global confusion on the diagnostic criteria for metabolic syndrome: what is the point that guidelines can not agree?. Anadolu Kardiyol Derg. 2008;8(2):149-153.
- Neeland IJ, Lim S, Tchernof A. Metabolic syndrome. Nat Rev Dis Primers. 2024;10(1):77-.
- Sperling LS, Mechanick JI, Neeland IJ. The cardiometabolic health alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66(9):1050-1067.
- Neeland IJ, Ayers CR, Rohatgi AK. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring). 2013;21(9):E439-E447.
- Chen O, Sharma A, Ahmad I. Correlation between pericardial, mediastinal, and intrathoracic fat volumes with the presence and severity of coronary artery disease, metabolic syndrome, and cardiac risk factors. Eur Heart J Cardiovasc Imaging. 2015;16(1):37-46.
- Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477-1500.
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359-404.
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338-349.
- Theodorakis N, Nikolaou M. Integrated management of cardiovascular-renal-hepatic-metabolic syndrome: expanding roles of SGLT2is, GLP-1RAs, and GIP/GLP-1RAs. Biomedicines. 2025;13(1):135-.
- Zinman B, Wanner C, Lachin JM. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
- Mahaffey KW, Neal B, Perkovic V. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study). Circulation. 2018;137(4):323-334.
- Anker SD, Butler J, Filippatos G. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461.
- Zannad F, Ferreira JP, Pocock SJ. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819-829.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446.
- Herrington WG, Staplin N, Wanner C. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127.
- Marso SP, Daniels GH, Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
- Marso SP, Bain SC, Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
- Lincoff AM, Brown-Frandsen K, Colhoun HM. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232.
- Kosiborod MN, Abildstrøm SZ, Borlaug BA. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069-1084.
- Perkovic V, Tuttle KR, Rossing P. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391(2):109-121.
- Sanyal AJ, Newsome PN, Kliers I. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392(21):2089-2099.
- Packer M, Zile MR, Kramer CM. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. 2025;392(5):427-437.
- Loomba R, Hartman ML, Lawitz EJ. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391(4):299-310.
- Solomon SD, McMurray JJV, Vaduganathan M. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2024;391(16):1475-1485.
- Vaduganathan M, Filippatos G, Claggett BL. Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes. Nat Med. 2024;30(12):3758-3764.
- Koskinas KC, Van Craenenbroeck EM, Antoniades C. Obesity and cardiovascular disease: an ESC clinical consensus statement. Eur J Prev Cardiol. 2025;32(3):184-220.
- Lüscher TF. Cardiometabolic medicine: the advent of systems cardiology and the new cardiovascular generalist?. Eur Heart J. ;():-.
- Rubino F, Cummings DE, Eckel RH. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025;13(3):221-262.
- Visseren FLJ, Mach F, Smulders YM. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337.
- Matsushita K, Jassal SK, Sang Y. Incorporating kidney disease measures into cardiovascular risk prediction: development and validation in 9 million adults from 72 datasets. EClinicalmedicine. 2020;27():100552-.
- Matsushita K, Kaptoge S, Hageman SHJ. Including measures of chronic kidney disease to improve cardiovascular risk prediction by SCORE2 and SCORE2-OP. Eur J Prev Cardiol. 2023;30(1):8-16.
- Rinella ME, Lazarus JV, Ratziu V. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542-1556.
- Eberly LA, Yang L, Eneanya ND. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4):e216139-.
- Eberly LA, Yang L, Essien UR. Racial, ethnic, and socioeconomic inequities in glucagon-like Peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12):e214182-.
- Sandhu AT, Heidenreich PA. The affordability of guideline-directed medical therapy: cost sharing is a critical barrier to therapy adoption. Circulation. 2021;143(11):1073-1075.
- Khan SS, Coresh J, Pencina MJ. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American Heart Association. Circulation. 2023;148(24):1982-2004.
- Massy ZA, Drueke TB. Combination of cardiovascular, kidney, and metabolic diseases in a syndrome named cardiovascular-kidney-metabolic, with new risk prediction equations. Kidney Int Rep. 2024;9(9):2608-2618.
- Khan SS, Breathett K, Braun LT. Risk-based primary prevention of heart failure: a scientific statement from the American Heart Association. Circulation. 2025;151(20):e1006-e1026.
- Busetto L, Dicker D, Frühbeck G. A new framework for the diagnosis, staging and management of obesity in adults. Nat Med. 2024;30(9):2395-2399.
- Khera AV, Emdin CA, Drake I. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349-2358.
- Depommier C, Everard A, Druart C. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096-1103.


